Question: How to compare slopes with the theoretical value base on constant value and ideal gas law ? Activity 1: Show plot of Volume vs. Temperature.
How to compare slopes with the theoretical value base on constant value and ideal gas law ?
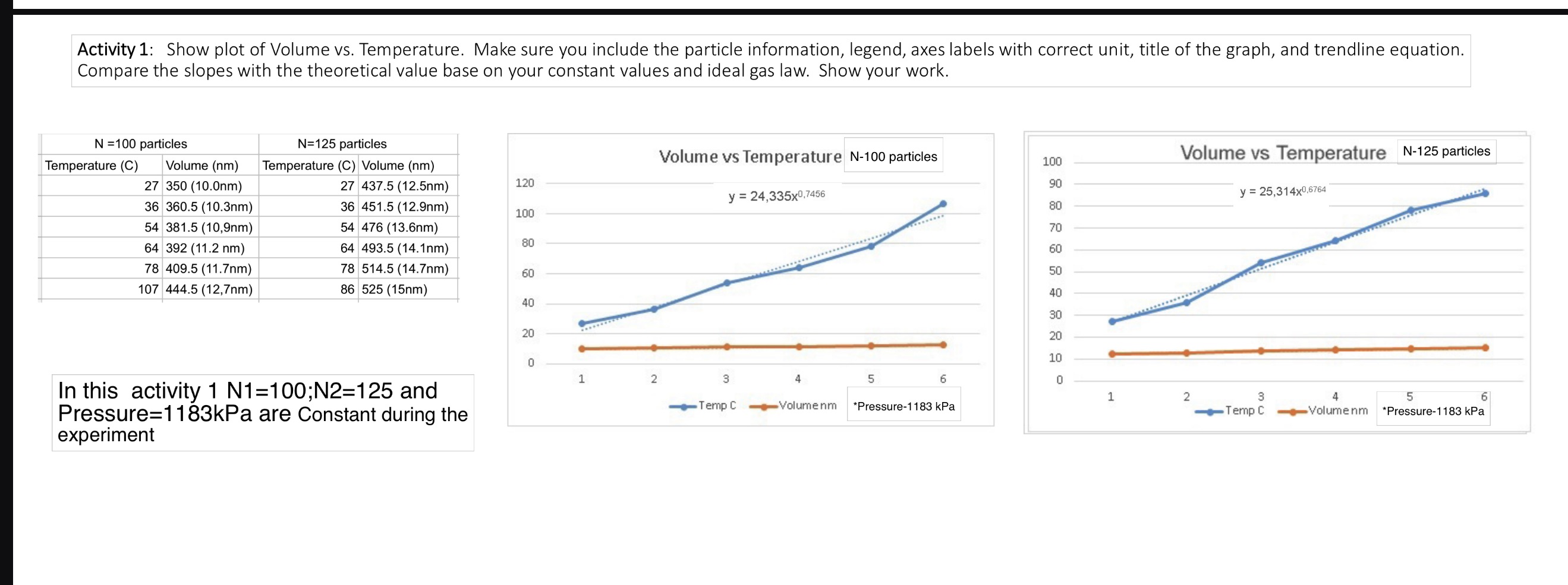
Activity 1: Show plot of Volume vs. Temperature. Make sure you include the particle information, legend, axes labels with correct unit, title of the graph, and trendline equation. Compare the slopes with the theoretical value base on your constant values and ideal gas law. Show your work. N=100 particles Temperature (C) Volume (nm) 27 350 (10.0nm) 36 360.5 (10.3nm) N=125 particles Temperature (C) Volume (nm). 27 437.5 (12.5nm) 36 451.5 (12.9nm) LL 54 381.5 (10,9nm) 64 392 (11.2 nm) 78 409.5 (11.7nm) 107 444.5 (12,7nm) 54 476 (13.6nm) 64 493.5 (14.1nm) 78 514.5 (14.7nm) 86 525 (15nm) In this activity 1 N1=100; N2=125 and Pressure=1183kPa are Constant during the experiment Volume vs Temperature N-100 particles 100 Volume vs Temperature N-125 particles 120 90 y 24,335x0,7456 y 25,314x0.6764 80 100 70 80 60 60 50 40 40 30 20 20 10 0 1 2 3 4 5 6 0 1 2 Temp C Volumenm *Pressure-1183 kPa 3 Temp C 4 Volumenm 5 6 *Pressure-1183 kPa
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


