Question: How to get the answer 0.091? For a diprotic acid HA, pKa, -4.00 and pka =9.00. What approximately would be the molar fraction of
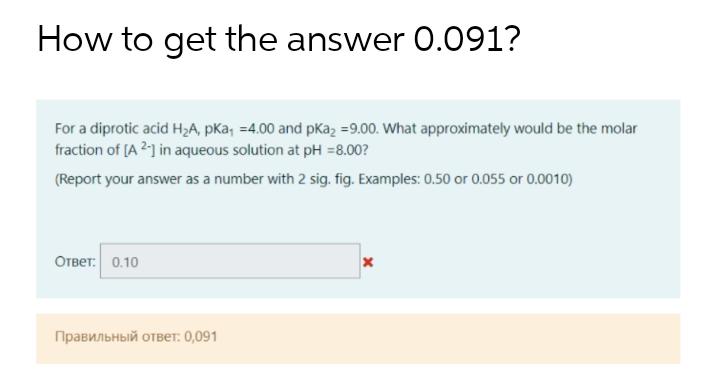
How to get the answer 0.091? For a diprotic acid HA, pKa, -4.00 and pka =9.00. What approximately would be the molar fraction of [A2-] in aqueous solution at pH = 8.00? (Report your answer as a number with 2 sig. fig. Examples: 0.50 or 0.055 or 0.0010) :0.10 : 0,091 x
Step by Step Solution
3.45 Rating (152 Votes )
There are 3 Steps involved in it
The detailed ... View full answer

Get step-by-step solutions from verified subject matter experts


