Question: how to solve a and d Note that the tie line intersects the solvus curve for solubility of Pb in Sn at a nonzero concentration
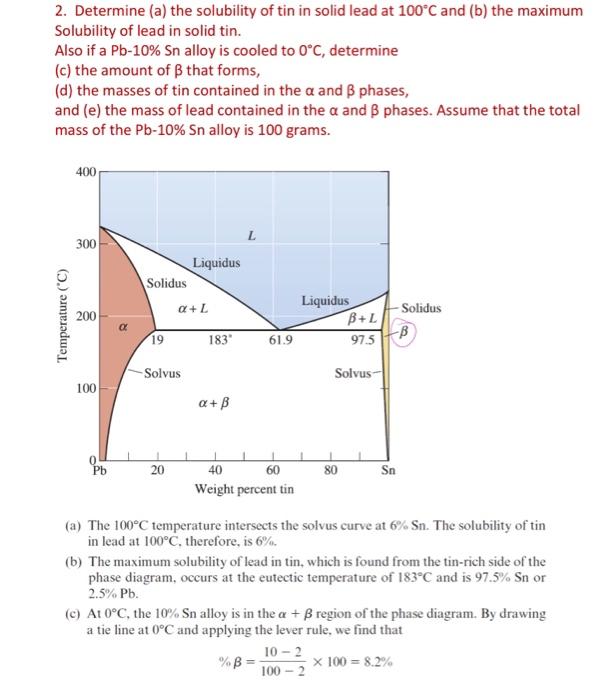
Note that the tie line intersects the solvus curve for solubility of Pb in Sn at a nonzero concentration of Sn. We cannot read this accurately from the diagram; thus, we assume that the right-hand point for the tie line is 100%Sn. The percent of would be (100%)=91.8%. This means if we have 100g of the 10%Sn alloy, it will consist of 8.2g of the phase and 91.8g of the phase. (d) Note that 100g of the alloy will consist of 10g of Sn and 90g of Pb. The Pb and Sn are distributed in two phases (i.e., and ). The mass of Sn in the phase =(2%Sn)(91.8g of phase )=(0.02)(91.8g)=1.836g. Since tin appears in both the and phases, the mass of Sn in the phase will be =(101.836)g=8.164g. Note that in this case, the phase at 0C is nearly pure Sn. (e) Let's now calculate the mass of lead in the two phases. The mass of Pb in the phase will be equal to the mass of the phase minus the mass of Sn in the phase =91.8g1.836g=89.964g. We could have also calculated this as MassofPbinthephase=(98%Pb)(91.8gofphase)=(0.98)(91.8g)=89.964g We know the total mass of the lead (90g), and we also know the mass of lead in the phase. Thus, the mass of Pb in the phase =9089.964=0.036g. This is consistent with what we said earlier (i.e., the phase, in this case, is almost pure tin). 2. Determine (a) the solubility of tin in solid lead at 100C and (b) the maximum Solubility of lead in solid tin. Also if a Pb10%Sn alloy is cooled to 0C, determine (c) the amount of that forms, (d) the masses of tin contained in the and phases, and (e) the mass of lead contained in the and phases. Assume that the total mass of the Pb10%Sn alloy is 100 grams. (a) The 100C temperature intersects the solvus curve at 6%Sn. The solubility of tin in lead at 100C, therefore, is 6%. (b) The maximum solubility of lead in tin, which is found from the tin-rich side of the phase diagram, occurs at the eutectic temperature of 183C and is 97.5%Sn or 2.5%Pb. (c) At 0C, the 10%Sn alloy is in the + region of the phase diagram. By drawing a tie line at 0C and applying the lever rule, we find that %=1002102100=8.2%
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


