Question: how to solve? Consider the following balanced equation: M3N2 + 6H20 - 3Mg(OH)2 + 2NH3 What mass of NH, can be formed from the reaction


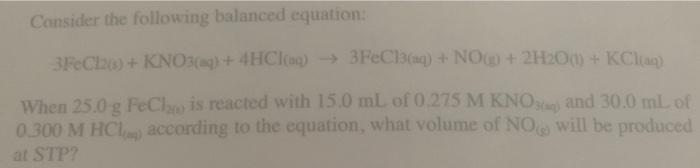
Consider the following balanced equation: M3N2 + 6H20 - 3Mg(OH)2 + 2NH3 What mass of NH, can be formed from the reaction of 35.0 g of Mg, N, with 55.0 g of H.O? (b) How many grams of the excess reactant will remain? walanced equation: RO+KO+ 4HCI > 3FeCl + NO + 2H2O + KCH is that of NO is produced when 65.2 g of FeCl, are reacted with 37.8 g of KNO and of HCl according to the equation. Consider the following balanced equation: 3FeCha+KNO3(aq) + 4HCI) 3FeCl3(aq) + NO + 2H200 + KCl) When 25.0 g FeCl is reacted with 15,0 mL of 0.275 M KNO3 and 30.0 mL of 0.300 M HCl. according to the equation, what volume of NO will be produced at STP
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


