Question: How to solve this 2. Turns out, the CO2 molecules in the atmosphere sometimes just fall apart. At pressure of P atmospheres, a certain fraction
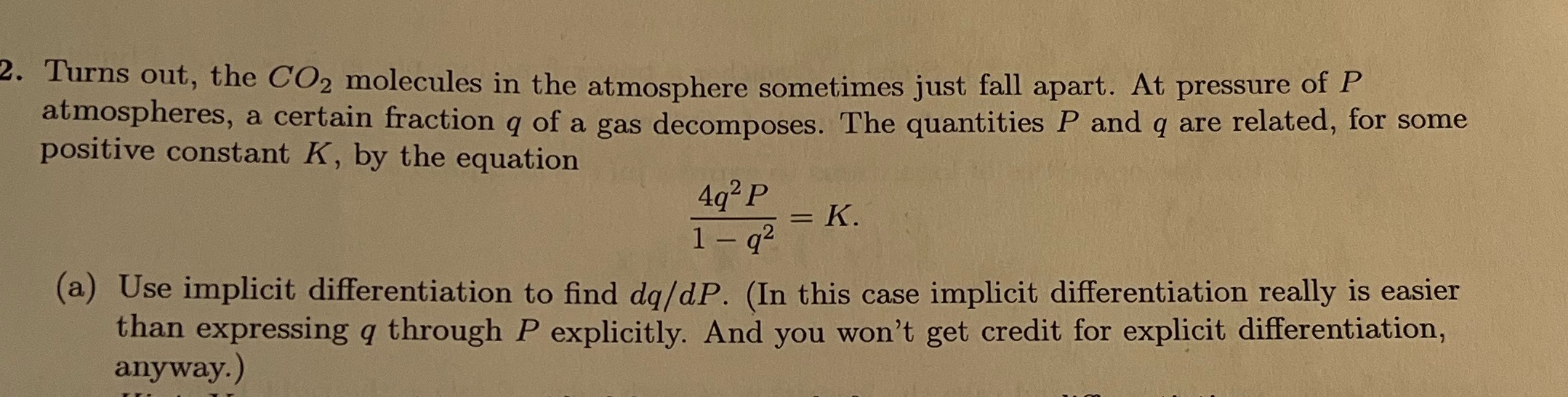
How to solve this
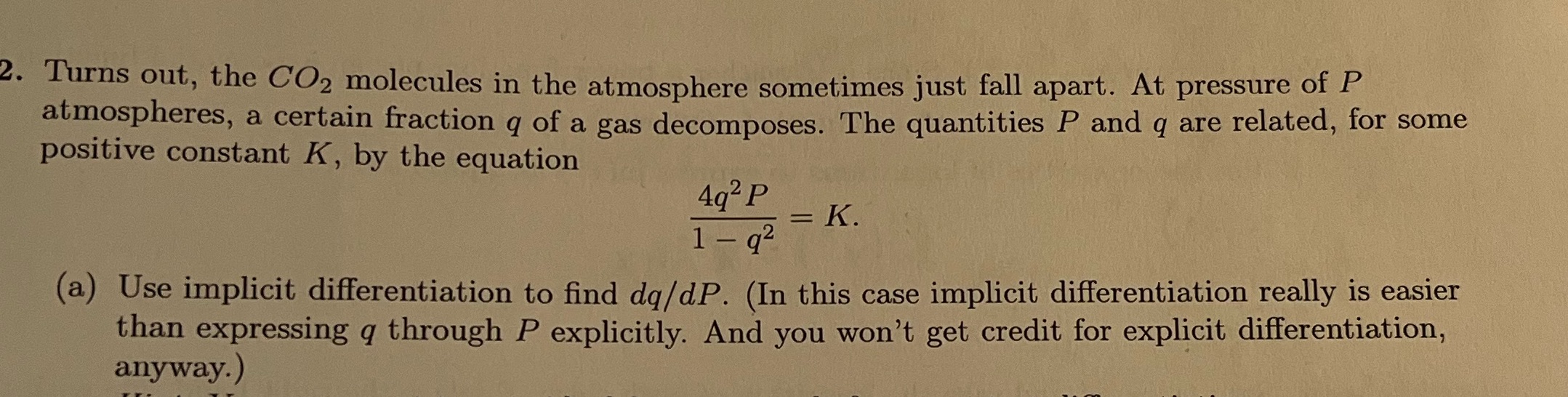
2. Turns out, the CO2 molecules in the atmosphere sometimes just fall apart. At pressure of P atmospheres, a certain fraction q of a gas decomposes. The quantities P and q are related, for some positive constant K, by the equation 492 P = K. 1 - q2 (a) Use implicit differentiation to find dq/dP. (In this case implicit differentiation really is easier than expressing q through P explicitly. And you won't get credit for explicit differentiation, anyway.)
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


