Question: how would i find the missing values? thank you! Values Obtained: (1) ppm CaCO3 (2) ppm CaCO3 (3) ppm CaCO 3 age value eviation Weight
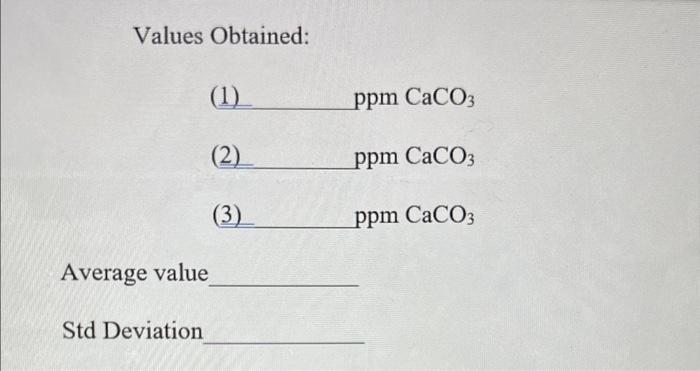
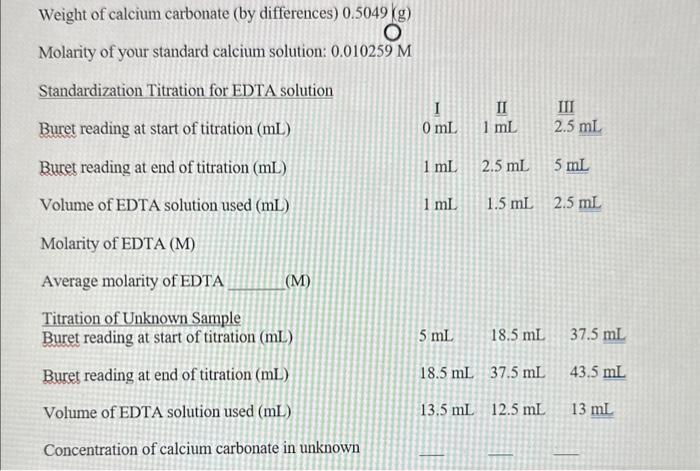

Values Obtained: (1) ppm CaCO3 (2) ppm CaCO3 (3) ppm CaCO 3 age value eviation Weight of calcium carbonate (by differences) 0.5049(g) Molarity of your standard calcium solution: 0.010259M Standardization Titration for EDTA solution \begin{tabular}{lr|r|r} Buret reading at start of titration (mL) & I & II & III \\ mL & 1mL & 2.5mL \end{tabular} Buret reading at end of titration (mL) 1mL2.5mL5mL Volume of EDTA solution used (mL) 1mL1.5mL2.5mL Molarity of EDTA (M) Average molarity of EDTA (M) Titration of Unknown Sample Buret reading at start of titration (mL) 5mL18.5mL37.5mL \begin{tabular}{l|lll} Buret reading at end of titration (mL) & 18.5mL & 37.5mL43.5mL \end{tabular} Volume of EDTA solution used (mL)13.5mL12.5mL13mL Concentration of calcium carbonate in unknown Sample calculations: FW of CaCO3=100.09g/mol=100.09mg/mmol;1ppm=1mg/L Let's say you titrate 25mL of unknown: ppmCaCO3=(25mLofukn)(103mL1L)(Msor)(VinmL)(mmol100.09mg) Values Obtained: (1) ppm CaCO3 (2) ppm CaCO3 (3) ppm CaCO 3 age value eviation Weight of calcium carbonate (by differences) 0.5049(g) Molarity of your standard calcium solution: 0.010259M Standardization Titration for EDTA solution \begin{tabular}{lr|r|r} Buret reading at start of titration (mL) & I & II & III \\ mL & 1mL & 2.5mL \end{tabular} Buret reading at end of titration (mL) 1mL2.5mL5mL Volume of EDTA solution used (mL) 1mL1.5mL2.5mL Molarity of EDTA (M) Average molarity of EDTA (M) Titration of Unknown Sample Buret reading at start of titration (mL) 5mL18.5mL37.5mL \begin{tabular}{l|lll} Buret reading at end of titration (mL) & 18.5mL & 37.5mL43.5mL \end{tabular} Volume of EDTA solution used (mL)13.5mL12.5mL13mL Concentration of calcium carbonate in unknown Sample calculations: FW of CaCO3=100.09g/mol=100.09mg/mmol;1ppm=1mg/L Let's say you titrate 25mL of unknown: ppmCaCO3=(25mLofukn)(103mL1L)(Msor)(VinmL)(mmol100.09mg)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


