Question: How would you interpret this equation? How would you interpret this equation? I have to find the limiting reagent so I can calculate theoretical yield
How would you interpret this equation? How would you interpret this equation? I have to find the limiting reagent so I can calculate theoretical yield and perform percent yield. I mixed g of CHNO gmol with g of NaSO gmol mL of M NaOH was added and stirred gently while placed in a hot sand bath for minutes. After minutes, the reaction is cooled at room temperature for minutes and then was added with mL of CHCOOH. The solidproduct formed is CHNO and was vacuum filtered.i aq
reflux
ii
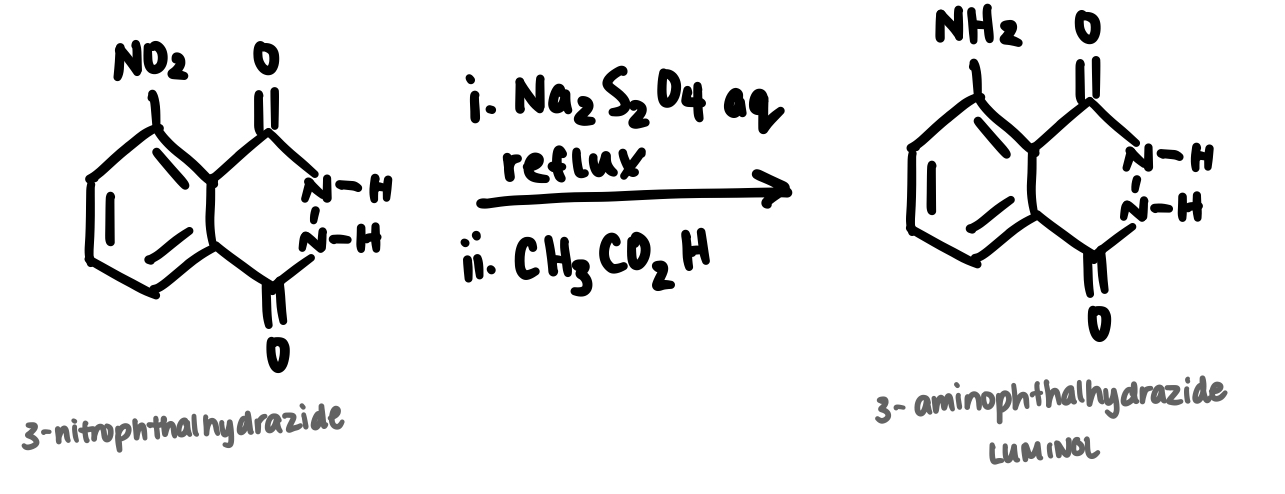
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


