Question: a) Two strains of Bacterium sweetans, STRI and STRII, use sucrose as a sole carbon source. The first step in the process of sucrose
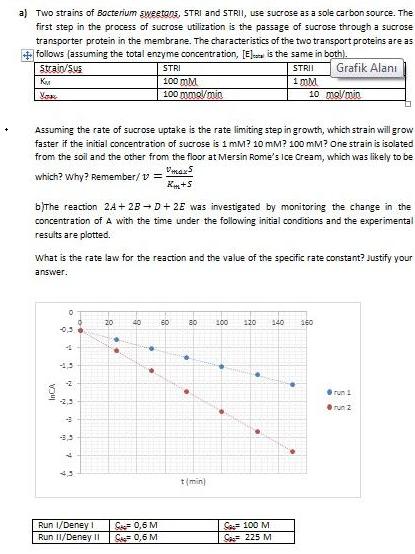
a) Two strains of Bacterium sweetans, STRI and STRII, use sucrose as a sole carbon source. The first step in the process of sucrose utilization is the passage of sucrose through a sucrose transporter protein in the membrane. The characteristics of the two transport proteins are as follows (assuming the total enzyme concentration, [El is the same in both). strain/Sus STRI STRII Grafik Alan 100 mM 100 mma/min 1 mM 10 mo/min Ve Assuming the rate of sucrose uptake is the rate limiting step in growth, which strain will grow faster if the initial concentration of sucrose is 1 mM? 10 mM? 100 mM? One strain is isolated from the soil and the other from the floor at Mersin Rome's Ice Cream, which was likely to be Vmaxs which? why? Remember/ v = K+5 bJThe reaction 2A+ 28 -D+ 2E was investigated by monitoring the change in the concentration ofA with the time under the following initial conditions and the experimental results are plotted. what is the rate law for the reaction and the value of the specific rate constant? Justify your answer. 20 40 60 80 100 120 140 160 run -2,5 Orun 2 43 t(min) S 0,6 M Sa 0,6 M Sa 100 M Ca 225 M Run 1/Deney i Run II/Deney II .. of a) Two strains of Bacterium sweetans, STRI and STRII, use sucrose as a sole carbon source. The first step in the process of sucrose utilization is the passage of sucrose through a sucrose transporter protein in the membrane. The characteristics of the two transport proteins are as follows (assuming the total enzyme concentration, [El is the same in both). strain/Sus STRI STRII Grafik Alan 100 mM 100 mma/min 1 mM 10 mo/min Ve Assuming the rate of sucrose uptake is the rate limiting step in growth, which strain will grow faster if the initial concentration of sucrose is 1 mM? 10 mM? 100 mM? One strain is isolated from the soil and the other from the floor at Mersin Rome's Ice Cream, which was likely to be Vmaxs which? why? Remember/ v = K+5 bJThe reaction 2A+ 28 -D+ 2E was investigated by monitoring the change in the concentration ofA with the time under the following initial conditions and the experimental results are plotted. what is the rate law for the reaction and the value of the specific rate constant? Justify your answer. 20 40 60 80 100 120 140 160 run -2,5 Orun 2 43 t(min) S 0,6 M Sa 0,6 M Sa 100 M Ca 225 M Run 1/Deney i Run II/Deney II .. of
Step by Step Solution
3.39 Rating (149 Votes )
There are 3 Steps involved in it
From given Km and Vmax data and micheal menton kinetic equation we know Km is ... View full answer

Get step-by-step solutions from verified subject matter experts


