Question: Hydrochloric acid solution test: Explain why when mixing these metals from the table below with HCI these changes occur: Metal Metal Reaction Evidence Gas
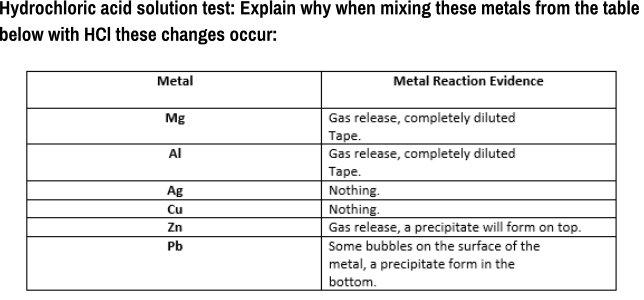
Hydrochloric acid solution test: Explain why when mixing these metals from the table below with HCI these changes occur: Metal Metal Reaction Evidence Gas release, completely diluted . Gas release, completely diluted Mg Al . Nothing. Ag Cu Nothing. Gas release, a precipitate will form on top. Zn Pb Some bubbles on the surface of the metal, a precipitate form in the bottom.
Step by Step Solution
3.42 Rating (152 Votes )
There are 3 Steps involved in it
Reaction of hydrochloric acid with magnesiumMg When hydrochloric acid reacts with magnesium ribbon magnesium chloride is produced and hydrogen gas is ... View full answer

Get step-by-step solutions from verified subject matter experts


