Question: Hydrogen can be produced as a co-product with styrene ( CsHs, a monomer for polystyrene polymer) by a catalytic dehydrogenation of ethylbenzene (CsH10) according to
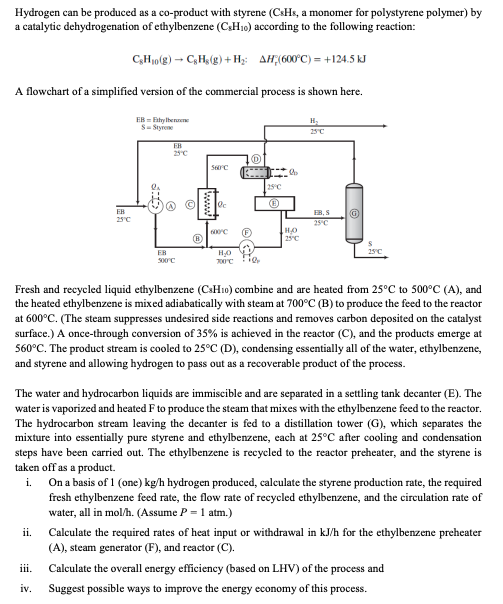
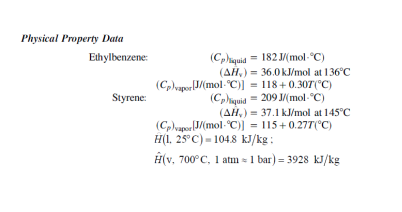
Hydrogen can be produced as a co-product with styrene ( CsHs, a monomer for polystyrene polymer) by a catalytic dehydrogenation of ethylbenzene (CsH10) according to the following reaction: C8H10(g)C8H8(g)+H2:Hr(600C)=+124.5kJ A flowchart of a simplified version of the commercial process is shown here. Fresh and recycled liquid ethylbenzene (CsH10) combine and are heated from 25C to 500C(A), and the heated ethylbenzene is mixed adiabatically with steam at 700C(B) to produce the feed to the reactor at 600C. (The steam suppresses undesired side reactions and removes carbon deposited on the catalyst surface.) A once-through conversion of 35% is achieved in the reactor (C), and the products emerge at 560C. The product stream is cooled to 25C (D), condensing essentially all of the water, ethylbenzene, and styrene and allowing hydrogen to pass out as a recoverable product of the process. The water and hydrocarbon liquids are immiscible and are separated in a settling tank decanter (E). The water is vaporized and heated F to produce the steam that mixes with the ethylbenzene feed to the reactor. The hydrocarbon stream leaving the decanter is fed to a distillation tower (G), which separates the mixture into essentially pure styrene and ethylbenzene, each at 25C after cooling and condensation steps have been carried out. The ethylbenzene is recycled to the reactor preheater, and the styrene is taken off as a product. i. On a basis of 1 (one) kg/h hydrogen produced, calculate the styrene production rate, the required fresh ethylbenzene feed rate, the flow rate of recycled ethylbenzene, and the circulation rate of water, all in mol/h. (Assume P=1 atm.) ii. Calculate the required rates of heat input or withdrawal in kJ/h for the ethylbenzene preheater (A), steam generator (F), and reactor (C). iii. Calculate the overall energy efficiency (based on LHV) of the process and iv. Suggest possible ways to improve the energy economy of this process. Physical Property Data Ethylbenzene:(Cp)Iquia=182J/(molC)(H~v)=36.0kJ/molat136C(CP)vapor[J/(molC)]=118+0.30T(C)(Cp)Iiquid=209J/(molC)(Hv)=37.1kJ/molat145C(Cp)vapos[J/(molC)]=115+0.27T(C)H(1,25C)=104.8kJ/kg:H^(v,700C,1atm1bar)=3928kJ/kg
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


