Question: Hydrogen, important for numerous processes, can be produced by the shift reaction: CO+ H20CO2+H2 In the reactor system shown in Figure P12.15, the conditions
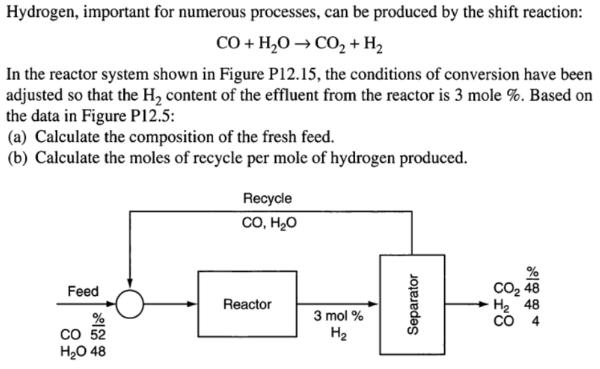
Hydrogen, important for numerous processes, can be produced by the shift reaction: CO+ H20CO2+H2 In the reactor system shown in Figure P12.15, the conditions of conversion have been adjusted so that the H2 content of the effluent from the reactor is 3 mole %. Based on the data in Figure Pl12.5: (a) Calculate the composition of the fresh feed. (b) Calculate the moles of recycle per mole of hydrogen produced Recycle , , CO2 48 H2 48 CO 4 Feed Reactor 3 mol % % CO 52 48 H2 Separator
Step by Step Solution
3.42 Rating (149 Votes )
There are 3 Steps involved in it
To solve this problem well perform a series of calculations based on the system and percentages prov... View full answer

Get step-by-step solutions from verified subject matter experts


