Question: Hydrogen peroxide, H 2 O 2 ( a a ) , must be stored at low temperatures to reduce the rate at which it decomposes
Hydrogen peroxide, must be stored at low temperatures to reduce the rate at which it decomposes to water and oxygen. The decomposition reaction is shown in the equation below.
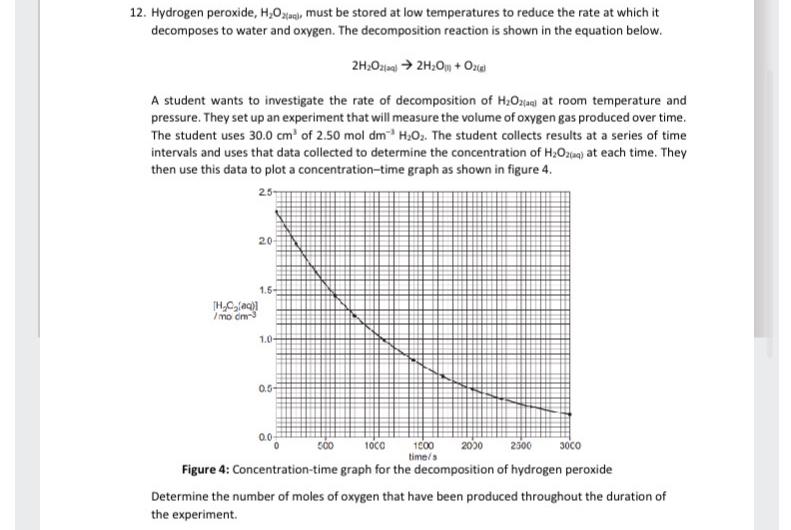
A student wants to investigate the rate of decomposition of at room temperature and pressure. They set up an experiment that will measure the volume of oxygen gas produced over time. The student uses of The student collects results at a series of time intervals and uses that data collected to determine the concentration of at each time. They then use this data to plot a concentrationtime graph as shown in figure
Figure : Concentrationtime graph for the decomposition of hydrogen peroxide
Determine the number of moles of oxygen that have been produced throughout the duration of the experiment.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


