Question: Hydrogen Spectrum Data Analysis: Be complete in your answers. For mathematical problems show your work with units and report answers to the correct number of
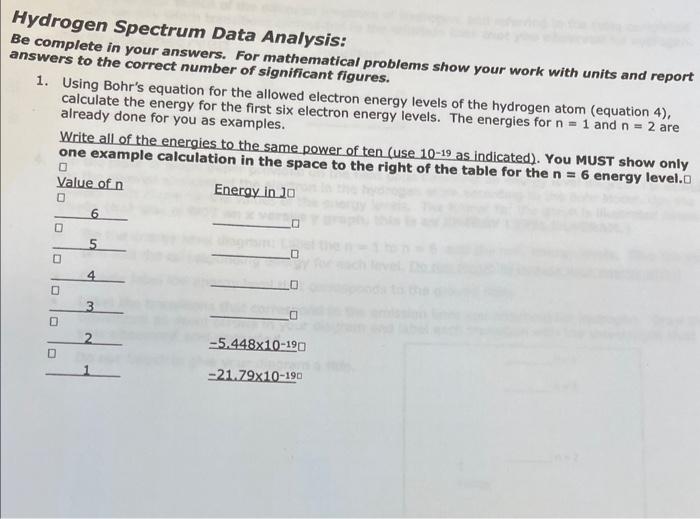
Hydrogen Spectrum Data Analysis: Be complete in your answers. For mathematical problems show your work with units and report answers to the correct number of significant figures. 1. Using Bohr's equation for the allowed electron energy levels of the hydrogen atom (equation 4), calculate the energy for the first six electron energy levels. The energies for n=1 and n=2 are already done for you as examples. Write all of the energies to the same power of ten (use 10-19 as indicated). You MuST show only one example calculation in the space to the right of the table for the n=6 energy level
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


