Question: I = 0 , k = 6 . 5 0 1 0 9 d m 3 m o l - 1 s - 1 I
When the subatomic species muonium Mu was first discovered in it was not known whether it bore an electric charge. The answer was provided by a kinetic study of the ionic strength effect on the reaction Mu Cu in aqueous solution. The following rate constants were measured at two ionic strengths:
beginarrayllI & ktimes mathrmdmmathrm~molmathrm~s I M & ktimes
Suppose that muonium had a single negative charge; what would k be expected to be at an ionic strength of M What do you deduce about the actual charge on muonium?
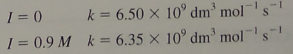
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


