Question: I am able to find the first two numbers but I don't understand how to do the last part. Please answer with a full explanation
I am able to find the first two numbers but I don't understand how to do the last part. Please answer with a full explanation and a correct answer!! :)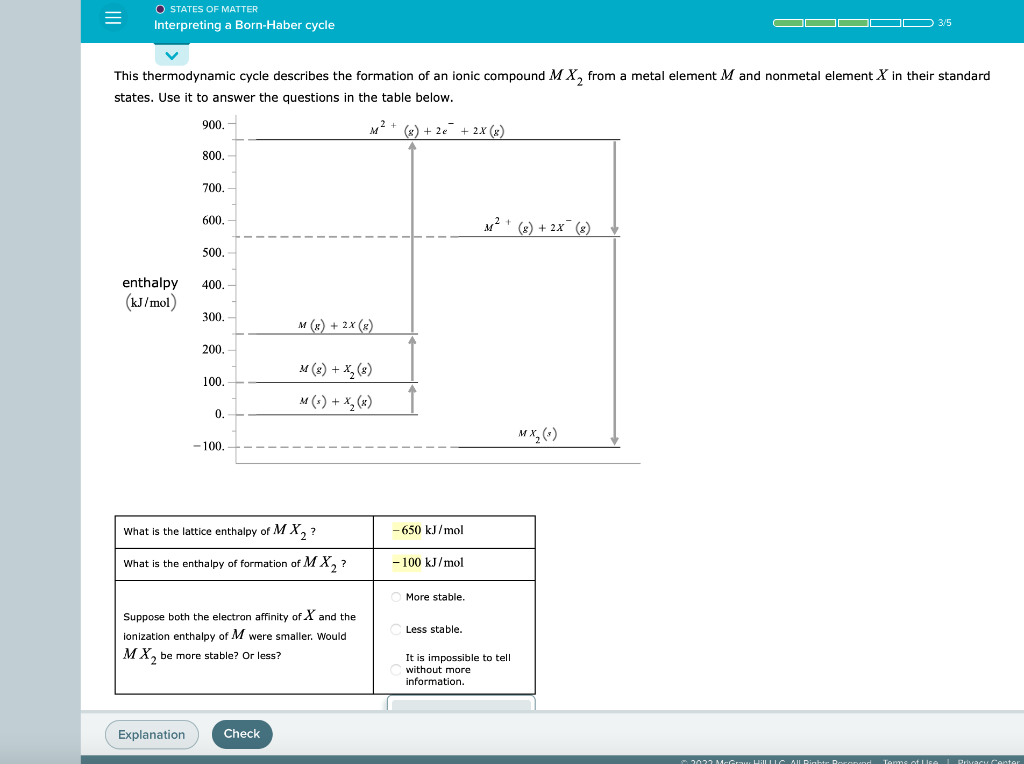
= O STATES OF MATTER Interpreting a Born Haber cycle COOOO 3/5 This thermodynamic cycle describes the formation of an ionic compound MX, from a metal element M and nonmetal element X in their standard states. Use it to answer the questions in the table below. 900.- M2 + (x) + 2 + 2x (3) 800. 700. 600. M2 + (8) + 2x (8) 500. 400. enthalpy (kJ/mol 300. M(x) + 2x) 200. 100. M(3) + X() M(-) + X(X) 0. mx) -100 - 650 kJ/mol What is the lattice enthalpy of MX,? What is the enthalpy formation of MX, -100 kJ/mol More stable. Less stable. Suppose both the electron affinity of X and the ionization enthalpy of M were smaller. Would MX, be more stable? Or less? It is impossible to tell without more information. Explanation Check 2022 Me
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


