Question: I am having some difficulty. Please show calculations. i also need help plotting the curve. I added a blank sheet which states the questions. For
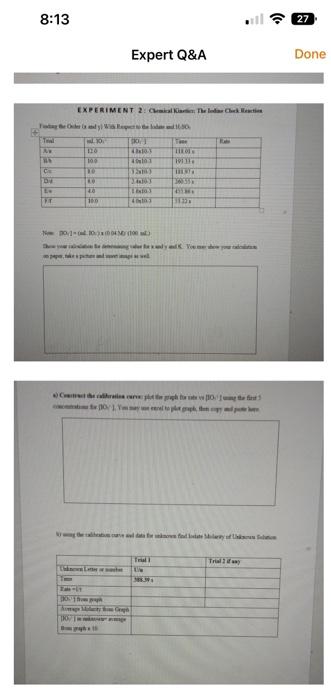


![) With Respect to the Iodate and H2SO3 Note: [IO3]=(mLIOs)(0.04M)(100mL) Show your](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66f8529882973_10466f8529808103.jpg)
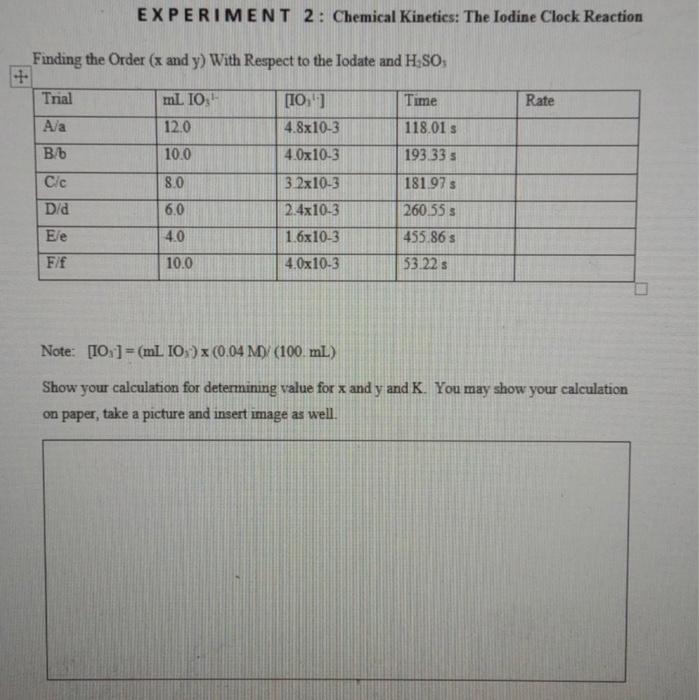
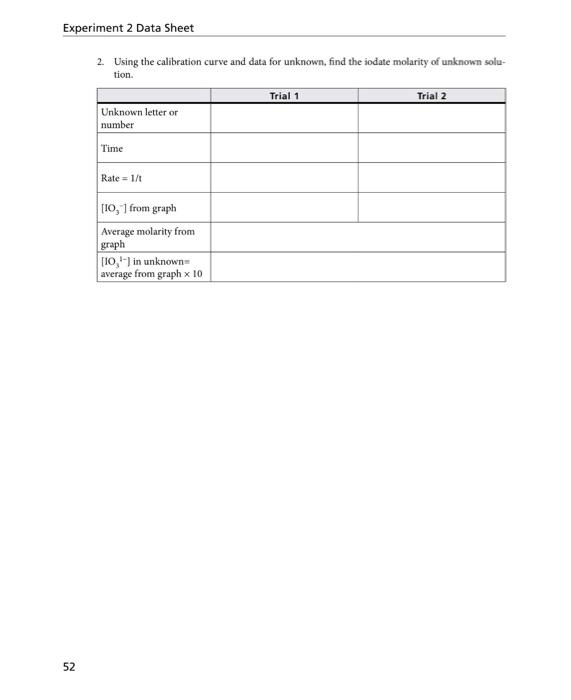
the mofa an morkingerverton wd) E X P E R I M E N T 2: Chemical Kinetics: The Iodine Clock Reaction Finding the Order ( x and y ) With Respect to the Iodate and H2SO3 Note: [IO3]=(mLIOs)(0.04M)(100mL) Show your calculation for determining value for x and y and K. You may show your calculation on paper, take a picture and insert image as well. a) Construct the calibration curve: plot the graph for rate vs [IO31] using the fint 5 concentrations for [IO1], You may use excel to plot graph, then copy and paste here. b) using the calibration curve and data for unknown find Iodate Molarity of Unknown Solution
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


