Question: I - ( aq ) + OCl - ( aq ) - - > IO - ( aq ) + Cl - ( aq )
Iaq OClaq IOaq Claq
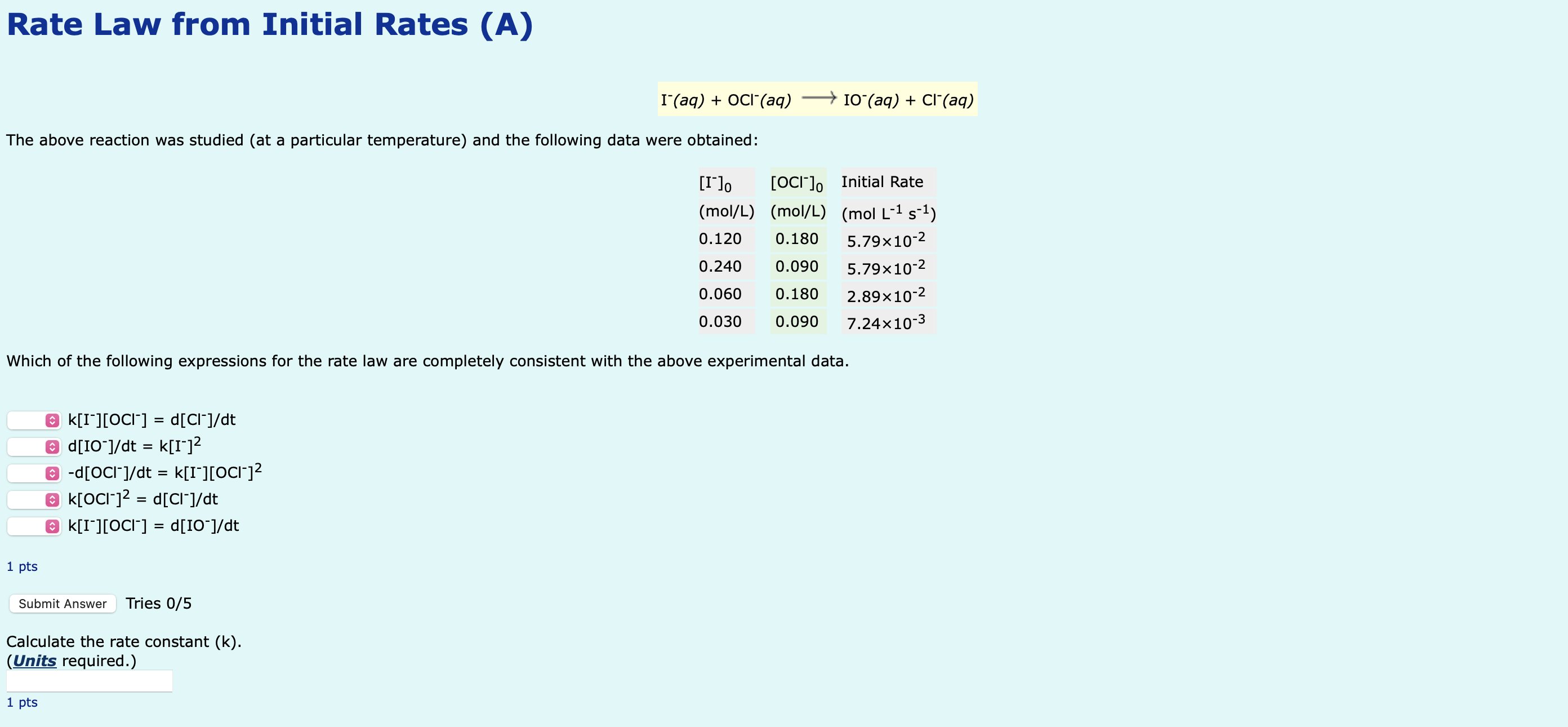
The above reaction was studied at a particular temperature and the following data were obtained:Which of thWhich of the following expressions for the rate law are completely consistent with the above experimental data.
kIOCl dCldt
dIOdt kI
dOCldt kIOCl
kOCl dCldt
kIOCl dIOdt
pts
Tries
Calculate the rate constant k
Units required.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


