Question: I asked this question, but I didn't understand the answer. I want a more detailed solution. 1. Nickel carbonyl (A) is produced by passing carbon
I asked this question, but I didn't understand the answer. I want a more detailed solution.
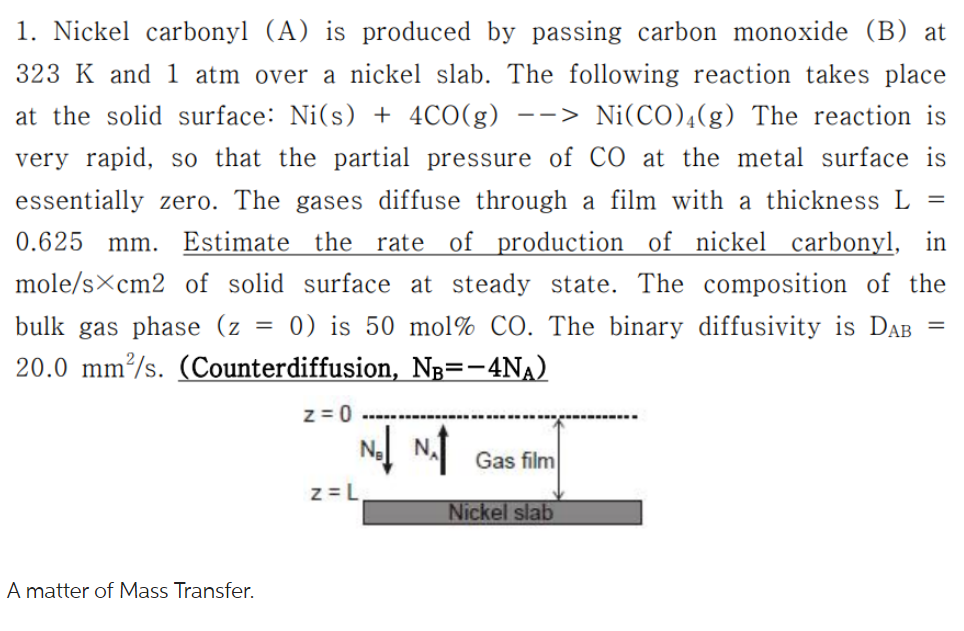
1. Nickel carbonyl (A) is produced by passing carbon monoxide (B) at 323K and 1 atm over a nickel slab. The following reaction takes place at the solid surface: Ni(s)+4CO(g)>Ni(CO)4(g) The reaction is very rapid, so that the partial pressure of CO at the metal surface is essentially zero. The gases diffuse through a film with a thickness L= 0.625mm. Estimate the rate of production of nickel carbonyl, in mole/scm2 of solid surface at steady state. The composition of the bulk gas phase (z=0) is 50mol% CO. The binary diffusivity is DAB= 20.0mm2/s. (Counterdiffusion, NB=4NA ) A matter of Mass Transfer. 1. Nickel carbonyl (A) is produced by passing carbon monoxide (B) at 323K and 1 atm over a nickel slab. The following reaction takes place at the solid surface: Ni(s)+4CO(g)>Ni(CO)4(g) The reaction is very rapid, so that the partial pressure of CO at the metal surface is essentially zero. The gases diffuse through a film with a thickness L= 0.625mm. Estimate the rate of production of nickel carbonyl, in mole/scm2 of solid surface at steady state. The composition of the bulk gas phase (z=0) is 50mol% CO. The binary diffusivity is DAB= 20.0mm2/s. (Counterdiffusion, NB=4NA ) A matter of Mass Transfer
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


