Question: (i). Determine the work required to compress methane from 1 atm to 100 atm at a constant temperature of 25 C using van der Waals
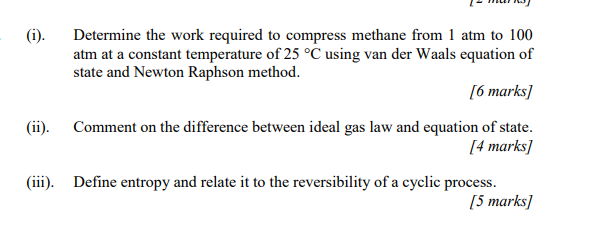
(i). Determine the work required to compress methane from 1 atm to 100 atm at a constant temperature of 25 C using van der Waals equation of state and Newton Raphson method. [6 marks] (ii). Comment on the difference between ideal gas law and equation of state. [4 marks] (iii). Define entropy and relate it to the reversibility of a cyclic process. (5 marks]
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


