Question: I didnt understand anything from this notes solve it again with clear steps please Water in the bottom of a uanow wetal tube is held
 I didnt understand anything from this notes solve it again with clear steps please
I didnt understand anything from this notes solve it again with clear steps please
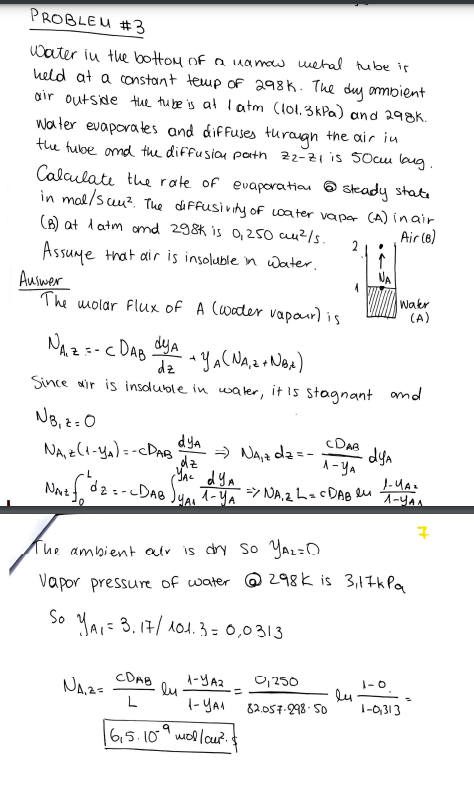
Water in the bottom of a uanow wetal tube is held at a constant temp of 298k. The dy ambient air outside the ture is at 1atm(101.3kPa ) and 298K. Water evaporates and diffuses turaugh the air in the tube and the diffusion path z2z1 is 50cm lang. Calculate the rate of evaporation 6 steady state in mal/scu2. The diffusivity of water vapar (A) in air. (B) at 1atm and 298K is 0,250cm2/s. Assume that air is insoluble in water. Auswer The molar flux of A (water vapaur) is NA,z=CDABdzdyA+yA(NA,z+NB,t) Since air is insoluble in water, it is stagnant and NB,z=0NA,z(1yA)=CDABdzdyANA,zdz=1yACDABdyANAt0Ldz=cDAByACyA11yAdyANA,2L=CDABlu1yA11yAz The ambient air is dry so yA2= Vapor pressure of water (a) 298K is 3,17kPa SoyA1=3.17/101.3=0,0313NA1z=LCDABln1yA11yA2=82.057.298500,250ln10,31310=
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


