Question: I don't know which one I got wrong (a) Given the following reactions and their heats of combustion, calculate the heat of combustion per CH
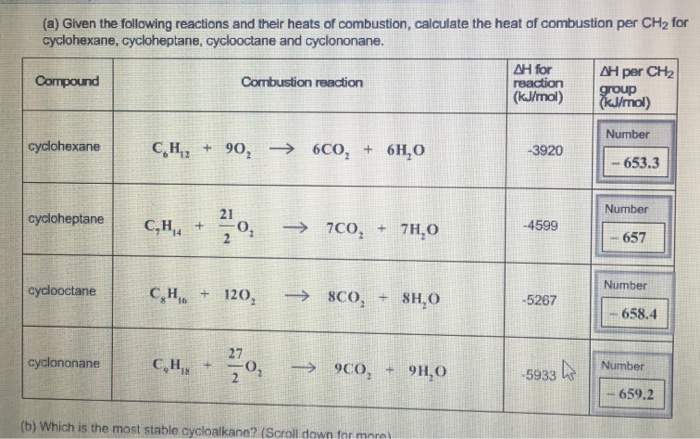
(a) Given the following reactions and their heats of combustion, calculate the heat of combustion per CH for cyclohexane, cycloheptane, cyclooctane and cyclononane. Compound cyclohexane cycloheptane cyclooctane cyclononane Combustion reaction CH, + 90, ) 6CO, + 6H,O 21 CH4 +0, CH + 120, C,H,, + 0, 7CO 7CO + 7HO SCO, + SHO 9CO, + 9H0 (b) Which is the most stable cycloalkane? (Scroll down for more AH for reaction (kJ/mol) -3920 -4599 -5267 -5933 AH per CH group (kJ/mol) Number -653.3 Number 657 Number 658.4 Number 659.2
Step by Step Solution
There are 3 Steps involved in it
To calculate ring strain in cyclic system angle strain and heat of combustions are used More the ang... View full answer

Get step-by-step solutions from verified subject matter experts


