Question: i dont really understand the charge and pH part here. because when I do it the COOH is already ionized and the charge is O.

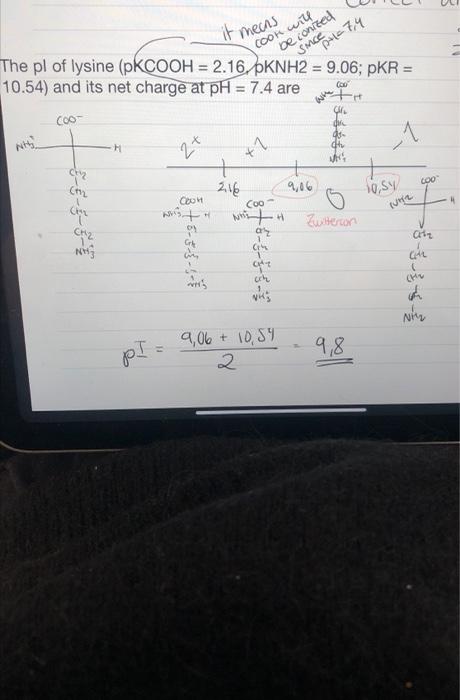
- A PI=9.8s, chasge on anvino said is +1 The pI of most arsine acid containing the ionizable sibe chain is average of pkavnlese of two similarly rouling group, at pH=7.4,2iaH will be ionized while anvino group is now-low/zed as PH
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


