Question: I finished my table, so can you help me with the questions 4 and 5, I really appreciated it! Data table: Boyle's Law Kaverage is
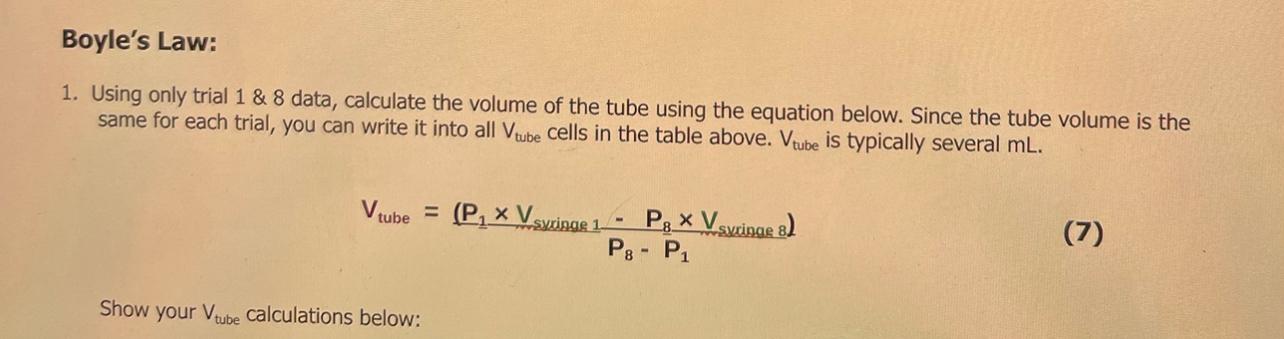
 I finished my table, so can you help me with the questions 4 and 5, I really appreciated it!
I finished my table, so can you help me with the questions 4 and 5, I really appreciated it!
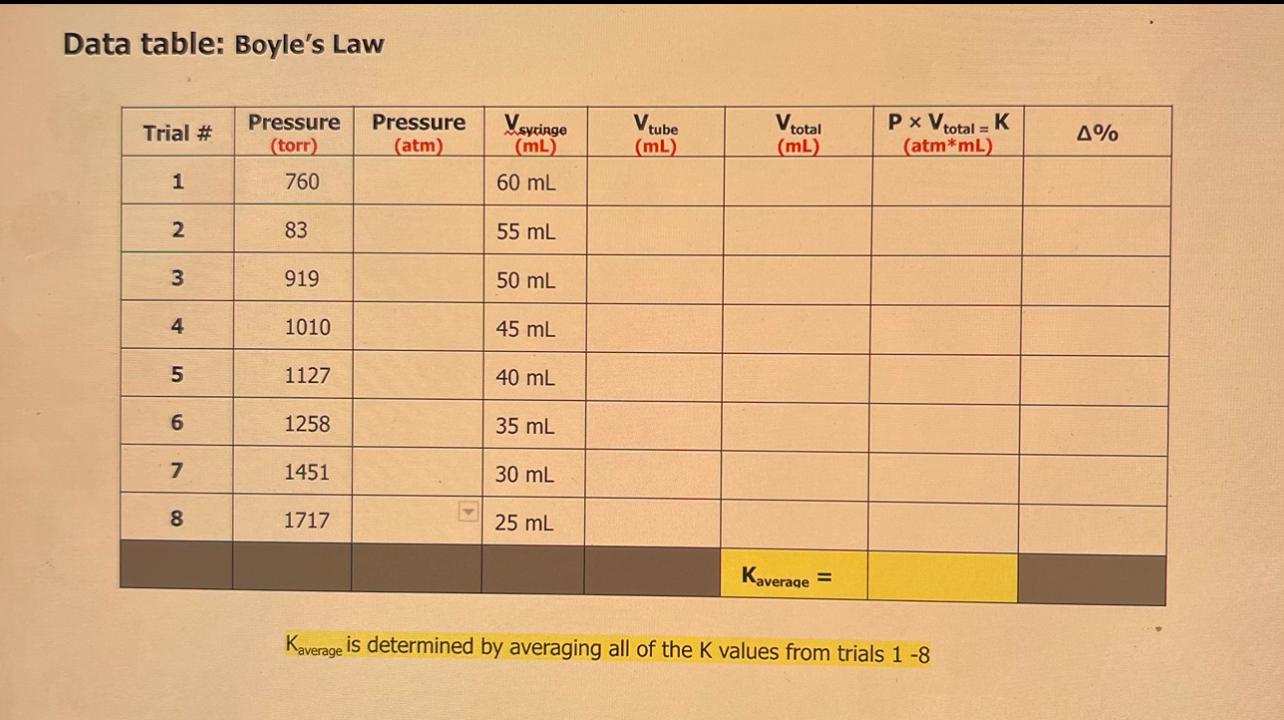
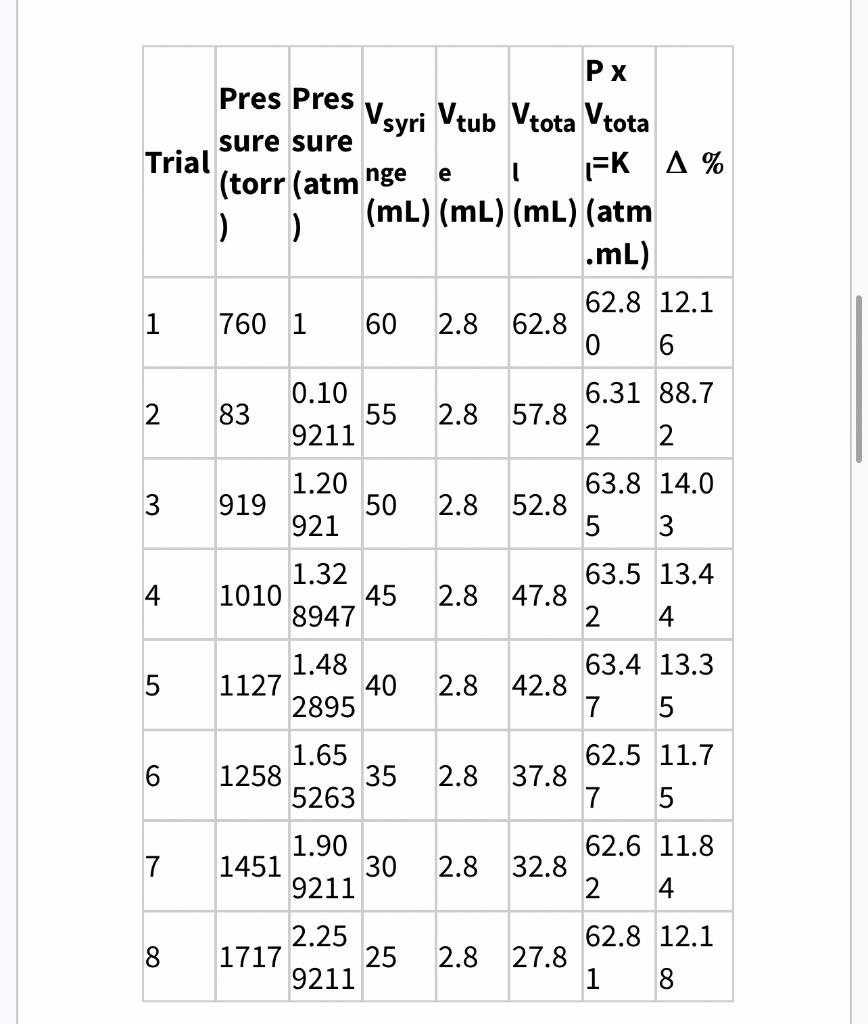
Data table: Boyle's Law Kaverage is determined by averaging all of the K values from trials 18 1. Using only trial 1 \& 8 data, calculate the volume of the tube using the equation below. Since the tube volume is the same for each trial, you can write it into all Vtube cells in the table above. Vtube is typically several mL. Vtube=P8P1(P1Vsyxizge1P8Vsucinae8) Show your Vtube calculations below: Calculate % for each trial and record each result in the data table above with 2 decimal point accuracy. Show one of your 10 calculations below. 5. Use your Average "K" value to determine the gas pressure you would expect to measure when the syringe is at the 3.0mL position in this experiment. Show your calculations below
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


