Question: i got them weong. please provide me with the correct answer. Thank you The pH of a solution is 11.95 +0.04. What is the concentration
i got them weong. please provide me with the correct answer. Thank you 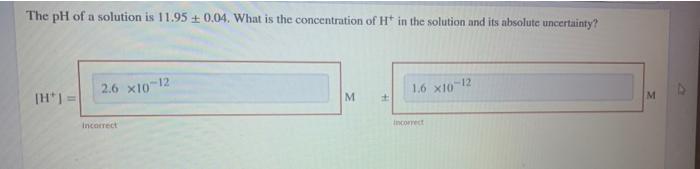
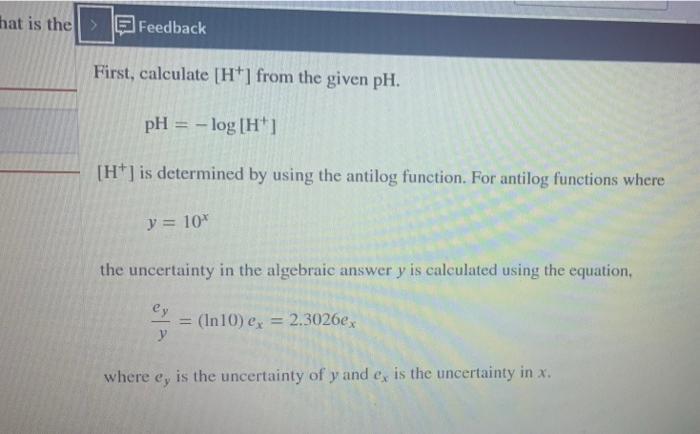


The pH of a solution is 11.95 +0.04. What is the concentration of Ht in the solution and its absolute uncertainty? 26 X101 12 1.6 x10-12 = | M + M Income incorrect that is the Feedback First, calculate [H] from the given pH. pH = -log[H" [H] is determined by using the antilog function. For antilog functions where y = 10% the uncertainty in the algebraic answer y is calculated using the equation, ey (In10) ex = 2.3026ex where ey is the uncertainty of y and er is the uncertainty in x
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


