Question: I have a material science problem, I will thumbs up if solved within 3 hours An iron-carbon alloy initially containing 0.284 wt% C is exposed
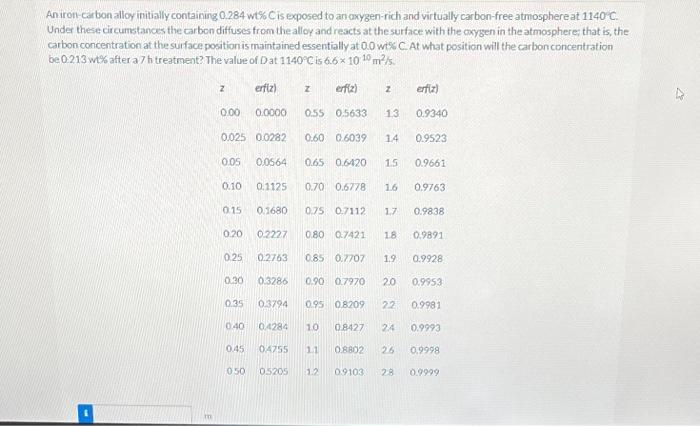
An iron-carbon alloy initially containing 0.284 wt\% C is exposed to an axygen-rich and virtually carbon-free atmosphereat 1140%C. Under these circumstances the carbon diffuses from the alloy and reacts at the surface with the cxygen in the atmosphere; that is, the carbon concentration at the surface position is maintained essentially at 0.0 w.5 C. At what position will the carbon concentration be 0.213wt% after a 7 h treatrent? The value of D at 1140C is 6.61010m2/5
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


