Question: i have one more try please help:) i also need my answer to 1 significant digit Incorrect Row 2: Your answer is wrong. In addition
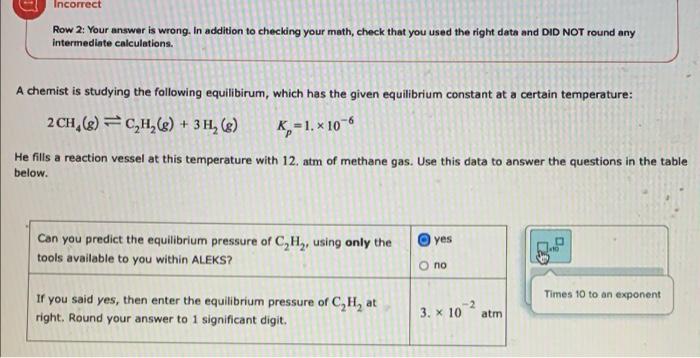
Incorrect Row 2: Your answer is wrong. In addition to checking your math, check that you used the right data and DID NOT round any intermediate calculations. A chemist is studying the following equilibirum, which has the given equilibrium constant at a certain temperature: 2CH (8) = C,H,($) + 3 H, ($) K=1.x 10-6 He fills a reaction vessel at this temperature with 12. atm of methane gas. Use this data to answer the questions in the table below. P yes Can you predict the equilibrium pressure of C,H, using only the tools available to you within ALEKS? 30 o no Times 10 to an exponent If you said yes, then enter the equilibrium pressure of C,H, at right. Round your answer to 1 significant digit. -2 3. X 10 atm
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


