Question: please help, i cant figure out what i did wrong incorrect Your answer is wrong in addition to checking your math, check that you used
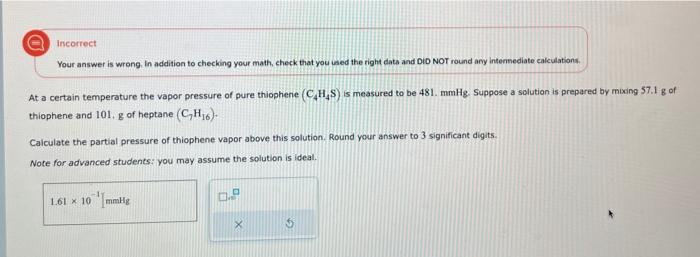
incorrect Your answer is wrong in addition to checking your math, check that you used the right date and DiD NOT round any internediate ealculations. At a certain temperature the vapor pressure of pure thiophene (C4H4S) is measured to be 481 . mmHg. Suppose a solution is prepared by mixing 57.1g of thiophene and 101,g of heptane (C7H16). Calculate the partial pressure of thiophene vapor above this solution. Round your answer to 3 significant digits. Note for advanced students: you may assume the solution is ideal
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


