Question: I Have solved everything until part d in question 6, but I am unsure how to do the rest. Table 1 tracks the composition of
I Have solved everything until part d in question 6, but I am unsure how to do the rest.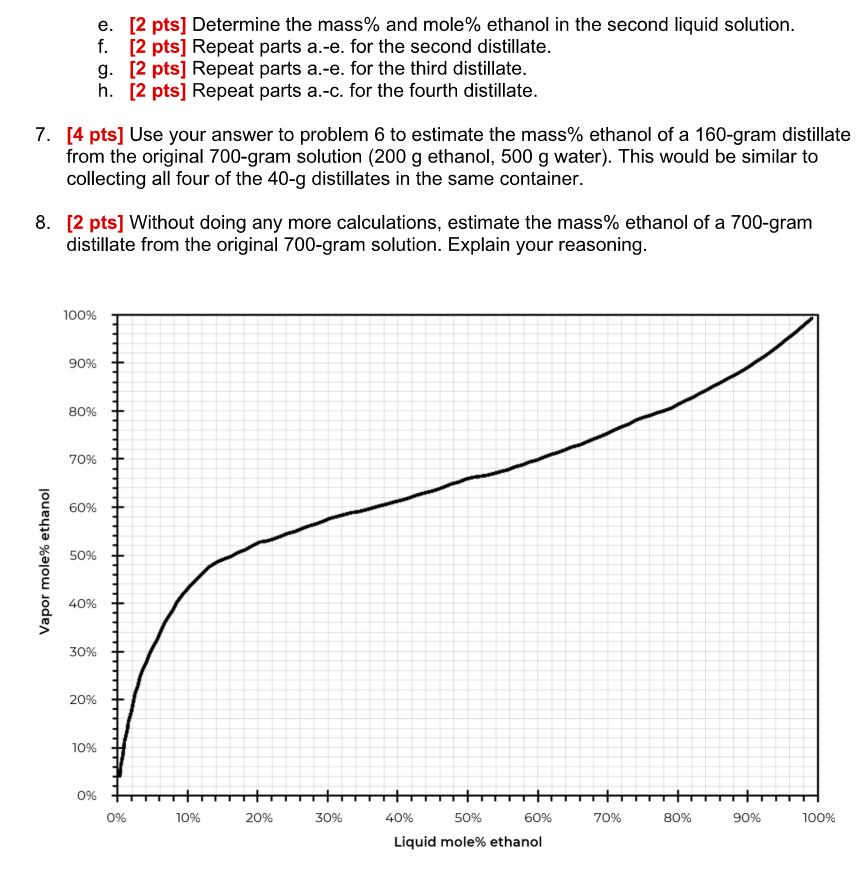
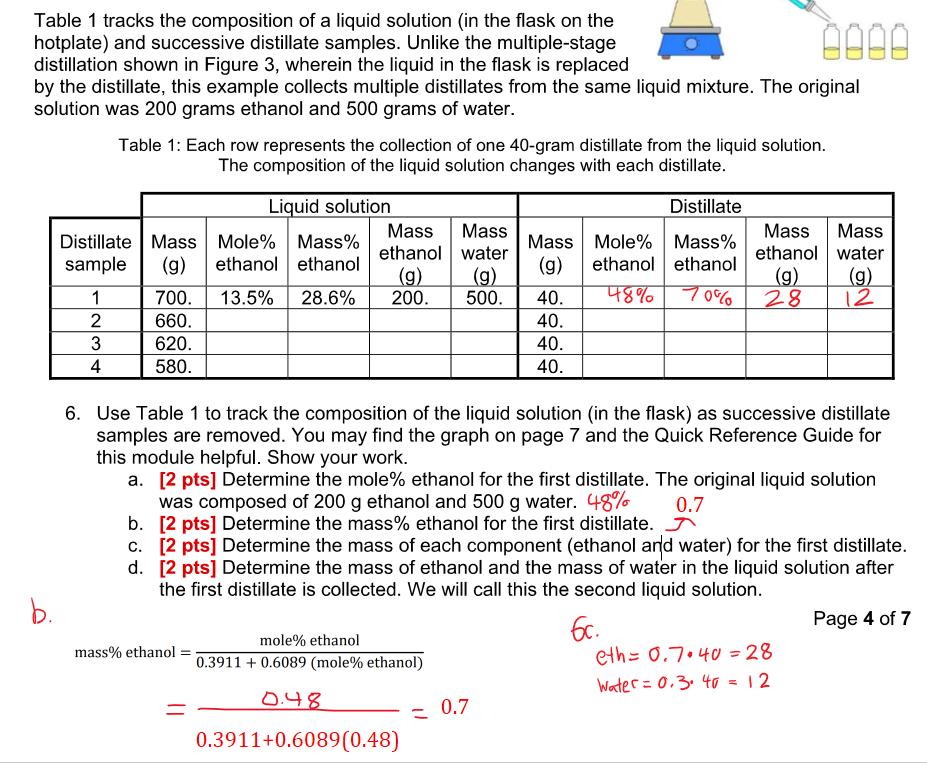
Table 1 tracks the composition of a liquid solution (in the flask on the hotplate) and successive distillate samples. Unlike the multiple-stage distillation shown in Figure 3, wherein the liquid in the flask is replaced by the distillate, this example collects multiple distillates from the same liquid mixture. The original solution was 200 grams ethanol and 500 grams of water. Table 1: Each row represents the collection of one 40-gram distillate from the liquid solution. The composition of the liquid solution changes with each distillate. Liquid solution Distillate Distillate Mass Mole% Mass% sample (g) ethanol ethanol 1 700. 13.5% Mass Mass ethanol water (g) 28.6% 200. Mass Mole% Mass% (g) ethanol ethanol Mass Mass ethanol water (g) (g) (g) 500. 40. 48% 70% 28 12 2 660. 40. 3 620. 40. 4 580. 40. b. 6. Use Table 1 to track the composition of the liquid solution (in the flask) as successive distillate samples are removed. You may find the graph on page 7 and the Quick Reference Guide for this module helpful. Show your work. a. [2 pts] Determine the mole% ethanol for the first distillate. The original liquid solution was composed of 200 g ethanol and 500 g water. 48% 0.7 b. [2 pts] Determine the mass% ethanol for the first distillate. c. [2 pts] Determine the mass of each component (ethanol and water) for the first distillate. d. [2 pts] Determine the mass of ethanol and the mass of water in the liquid solution after the first distillate is collected. We will call this the second liquid solution. mass% ethanol = mole% ethanol 0.3911 +0.6089 (mole% ethanol) 0.48 0.3911+0.6089(0.48) 0.7 6c. Page 4 of 7 eth0.7.40 = 28 Water 0.3. 40 = 12
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


