Question: i have the answer but i need the steps plz 1kmol/h of a mixture of two components (x1=0.5, separation factor 2.5) is isothermally flashed into
i have the answer but i need the steps plz
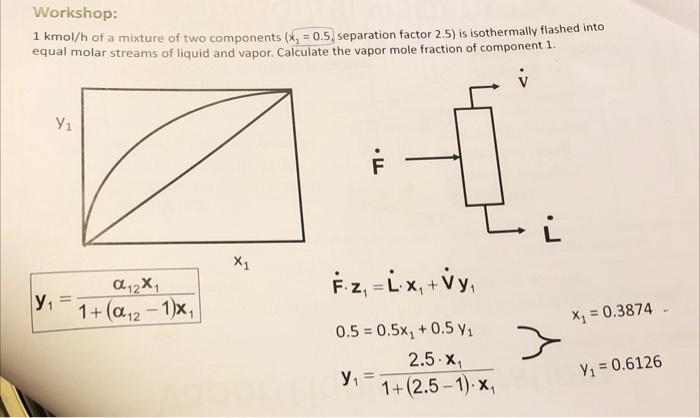
1kmol/h of a mixture of two components (x1=0.5, separation factor 2.5) is isothermally flashed into equal molar streams of liquid and vapor. Calculate the vapor mole fraction of component 1. y1=1+(121)x112x1 x1Fz1=Lx1+Vy10.5=0.5x1+0.5y1y1=1+(2.51)x12.5x1y1=0.6126
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


