Question: I have tried to solve it but ibkeep getting it wrong, please help me solve it step by step so I can understand the process
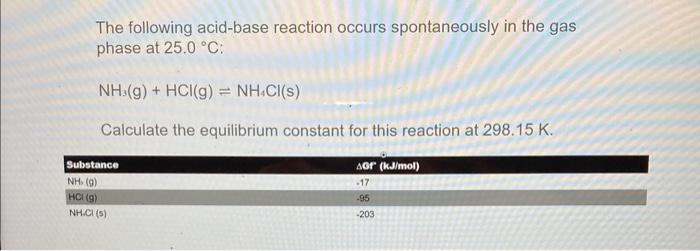
The following acid-base reaction occurs spontaneously in the gas phase at 25.0C : NH3(g)+HCl(g)NH4Cl(s) Calculate the equilibrium constant for this reaction at 298.15K
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


