Question: I I need help with the first part and is the 2nd part correct? Determination Of Solubility Product Constant Data Collection Concentration of standard HCl





 I I need help with the first part and is the 2nd part correct?
I I need help with the first part and is the 2nd part correct?
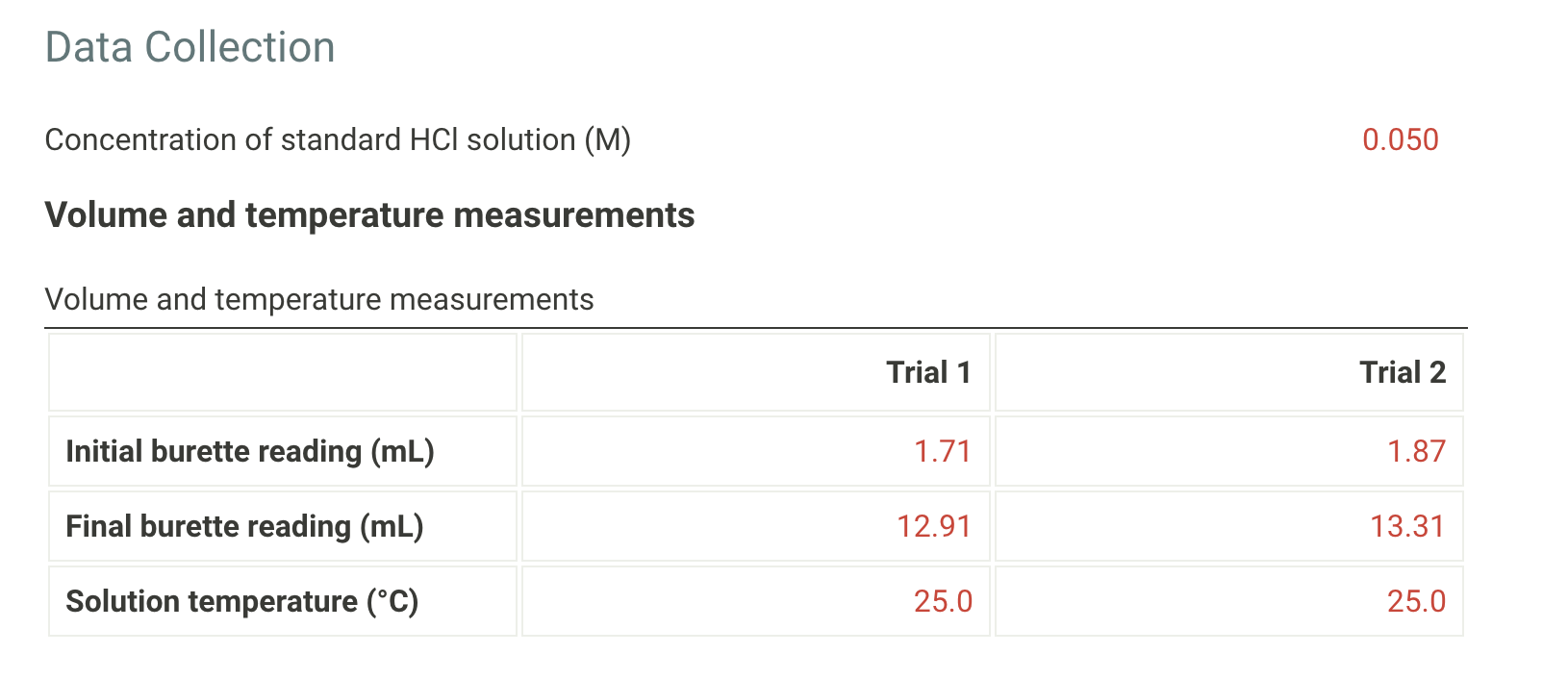
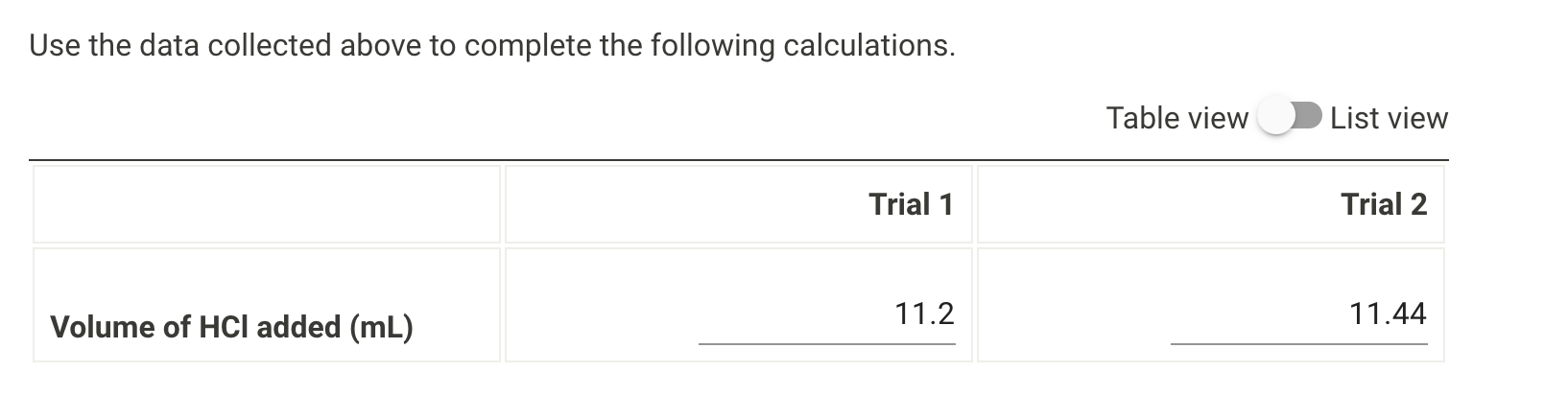
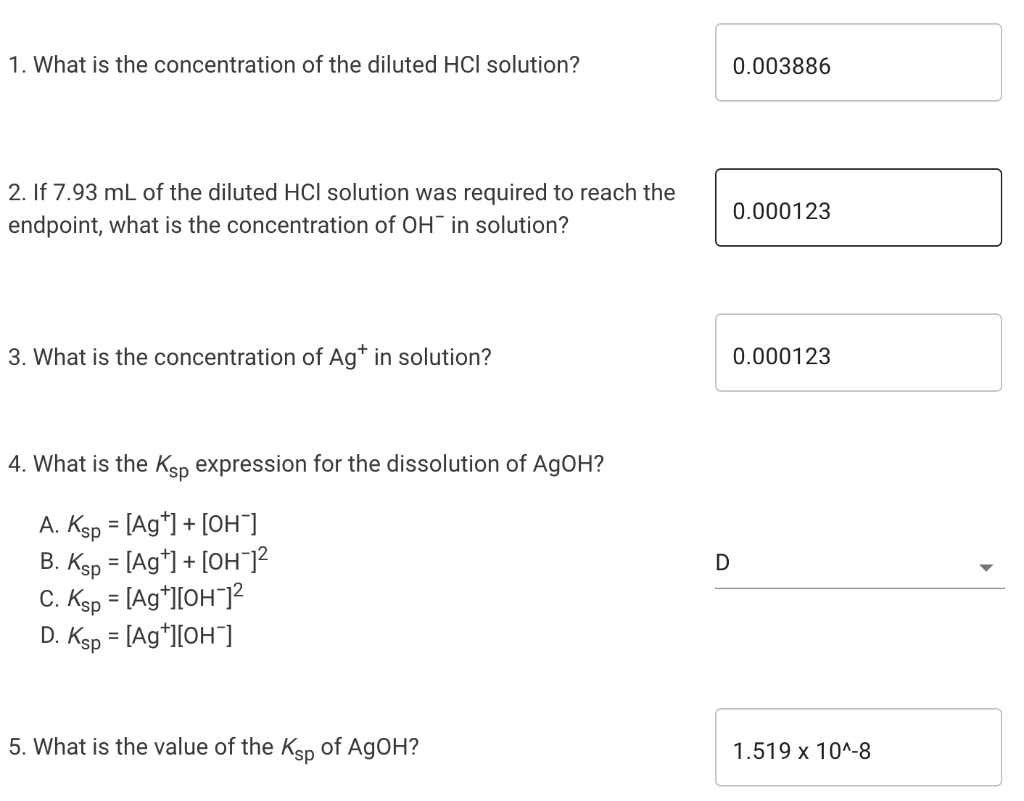
Determination Of Solubility Product Constant Data Collection Concentration of standard HCl solution (M) 0.050 Volume and temperature measurements Volume and temperature measurements Trial 1 Trial 2 Initial burette reading (mL) 1.71 1.87 Final burette reading (mL) 12.91 13.31 Solution temperature (C) 25.0 25.0 Calculations Use the data collected above to complete the following calculations. Table view List view Trial 1 Trial 2 Volume of HCl added (mL) 11.2 11.44 Average volume HCl added (mL) 11.32 Concentration of OH (M) Concentration of Ca2+ (M) Value of Ksp for Ca(OH)2 An HCl solution has a concentration of 0.09714 M. Then 10.00 mL of this solution was then diluted to 250.00 mL in a volumetric flask. The diluted solution was then used to titrate 250.0 mL of a saturated AGOH solution using methyl orange indicator to reach the endpoint. 1. What is the concentration of the diluted HCl solution? 0.003886 2. If 7.93 mL of the diluted HCl solution was required to reach the endpoint, what is the concentration of OH in solution? 0.000123 3. What is the concentration of Agt in solution? 0.000123 4. What is the Ksp expression for the ution of AgOH? = D A. Ksp = [Ag+] + [OH) B. Ksp = [Ag+] + [OH-]2 C. Ksp = [Ag+][OH-]2 D. Ksp = [Ag+][OHT] 5. What is the value of the Ksp of AgOH? 1.519 X 10^-8
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


