Question: i just need help with questions C, D, E please C (F - 32) 5/9 F=Cx 95 +32 Celsius to Kelvin (K) conversion K-C +
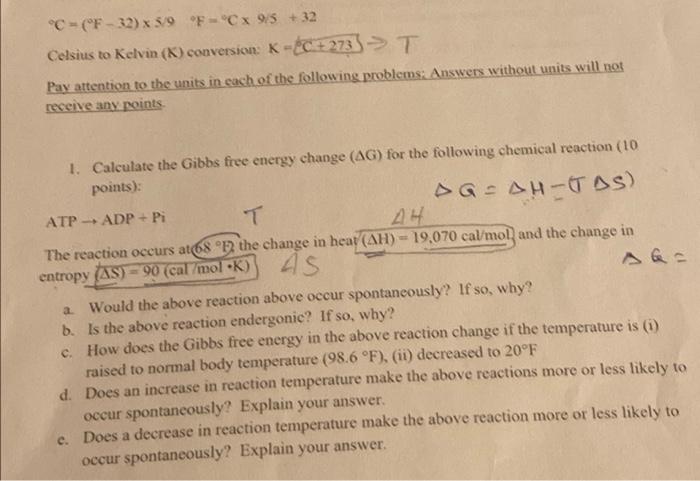
C (F - 32) 5/9 F="Cx 95 +32 Celsius to Kelvin (K) conversion K-C + 273) > T (: ) Pay attention to the units in each of the following problems: Answers without units will not receive any points As 1. Calculate the Gibbs free energy change (AG) for the following chemical reaction (10 points): AGAH-TAS) ATP-ADP + Pi T AH The reaction occurs at 68 F the change in heat (AH) - 19,070 cal/mol and the change in entropy (AS) = 90 (cal/molK) a Would the above reaction above occur spontaneously? If so, why? b. Is the above reaction endergonic? If so, why? c. How does the Gibbs free energy in the above reaction change if the temperature is (1) raised to normal body temperature (98.6 F), (ii) decreased to 20F d. Does an increase in reaction temperature make the above reactions more or less likely to occur spontaneously? Explain your answer. c. Does a decrease in reaction temperature make the above reaction more or less likely to occur spontaneously? Explain your
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


