Question: I JUST NEED INPUT ON THE RED PART BELOW (SEE THE SECOND PICTURE) .... Thumbs down if you just re-solve it. I worked out most
I JUST NEED INPUT ON THE RED PART BELOW (SEE THE SECOND PICTURE).... Thumbs down if you just re-solve it. I worked out most of the answer so far - and I get it-- but I don't understand the red part. Because how I'd think to do in algebraically would be:
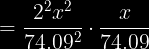
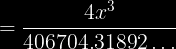
and then you would multiply by 406704.31892 on both sides, divide by 4, then take the cube root of the answer. But I get .823873425 on my calculator- not 2.34g.
Can someone explain that part to me?
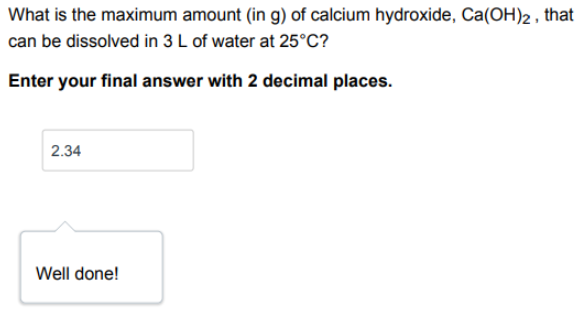
What is the maximum amount (in g) of calcium hydroxide, Ca(OH)_2, that can be dissolved in 3 L of water at 25C? THE ANSWER is 2.34

What is the maximum amount (in g) of calcium hydroxide, Ca(OH)2, that can be dissolved in 3L of water at 25C ? Enter your final answer with 2 decimal places. Solution: We can calculate the maximum amount of calcium hydroxide that can be dissolved in 3L of water at 25C using the solubility product constant (Ksp) for Ca(OH)2 at this temperature. The Ksp expression for Ca(OH)2 is : Ca(OH)2(s)Ca2+(aq)+2OH(aq)Ksp=[Ca2+][OH]2 Explanation: At 25C, the Ksp for Ca(OH)2 is 5.5106 Let x be the amount ( in g ) of Ca(OH)2 that dissolved in 3L of water Step 2/2 The molar mass of Ca(OH)2 is 74.09g/mol, so xg of the Ca(OH)2 corresponds to x/74.09mol of Ca(OH)2 The concentration of Ca2+joins and OHions in a saturated solution of Ca(OH)2 id the same and can be calculated as; [Ca2+]=74.09xmol/L[OH]=74.092xmol/L Substituting these concentrations into the Ksp expression, we get : Ksp=[Ca2][OH]2>5.5106=(74.09x)(74.092x)2 After calculation we get: x=2.34g
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


