Question: I JUST NEED PART C THANK YOU:) Problem 2 Before we get to the conversion of alumina to sodium aluminate, we must first deal with
I JUST NEED PART "C" THANK YOU:)
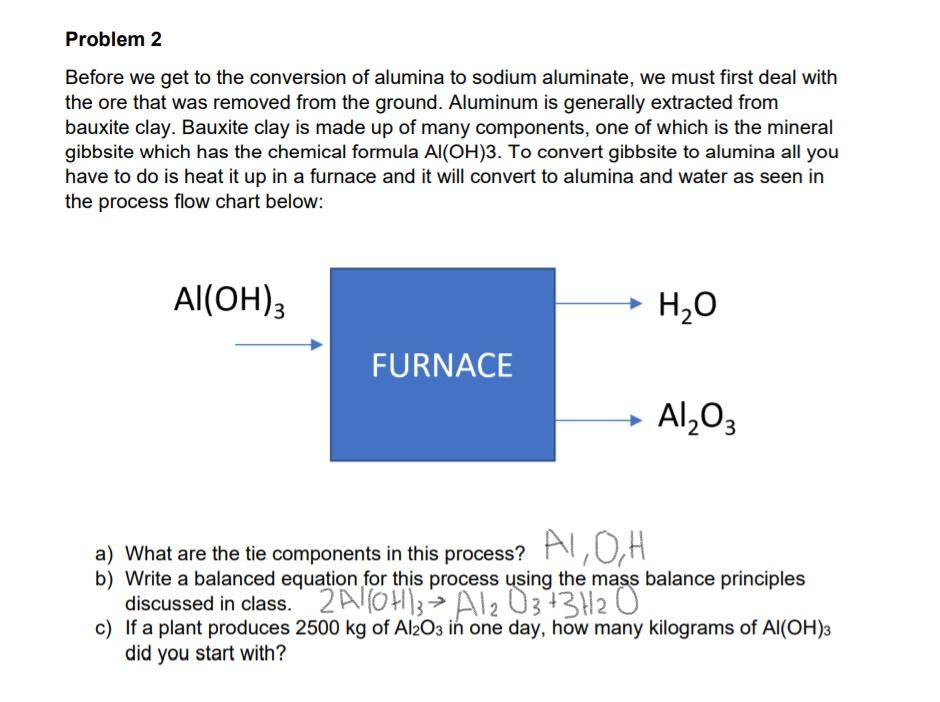
Problem 2 Before we get to the conversion of alumina to sodium aluminate, we must first deal with the ore that was removed from the ground. Aluminum is generally extracted from bauxite clay. Bauxite clay is made up of many components, one of which is the mineral gibbsite which has the chemical formula Al(OH)3. To convert gibbsite to alumina all you have to do is heat it up in a furnace and it will convert to alumina and water as seen in the process flow chart below: Al(OH) H2O FURNACE Al2O3 a) What are the tie components in this process? AL, OH b) Write a balanced equation for this process using the mass balance principles discussed in class. 2Al(OH), Al2O3+3H2O c) If a plant produces 2500 kg of Al2O3 in one day, how many kilograms of Al(OH)3 did you start with
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


