Question: i keep on getting a different answer. what would the solution be using this equation for part a? I might be getting the ionization energy

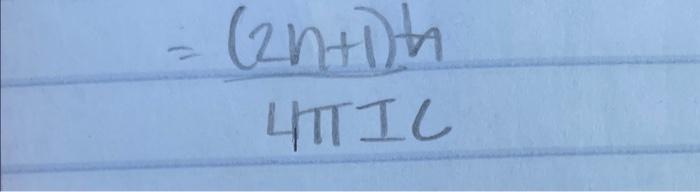
1. The wavelength of visible light ranges from 400nm to 700nm. Wavelengths just below 400nm are said to the in the ultraviolet, whereas wavelengths above 700 nm are said to be in the infrared. (a) The -electrons in benzene absorb light at a wavelength of 204nm. Assuming that the -electrons can be treated as quantum particles confined to a ring, what is the diameter of the ring? Remember: There are six electrons in a benzene ring. (b) How does your result compare with the known length of the CC bond in benzene? =(2n+1)n UIIIC
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


