Question: For the reaction NH4* (aq) + NO2 (aq)-> N2(g) + 2HO(g), if water is being produced at a rate of 0.765 M/s, at what
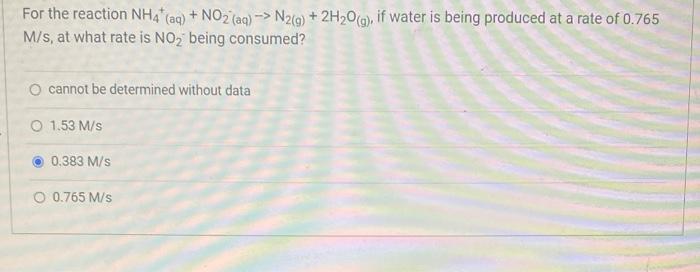
For the reaction NH4* (aq) + NO2 (aq)-> N2(g) + 2HO(g), if water is being produced at a rate of 0.765 M/s, at what rate is NO being consumed? O cannot be determined without data O 1.53 M/s 0.383 M/s O 0.765 M/s
Step by Step Solution
3.36 Rating (159 Votes )
There are 3 Steps involved in it
The detailed answer for the above question is provided below The balanced chemical equat... View full answer

Get step-by-step solutions from verified subject matter experts


