Question: I need a rule for Step 6 and based on it can you state for each drawing if its center, plane, or enantiomer. Step 6:
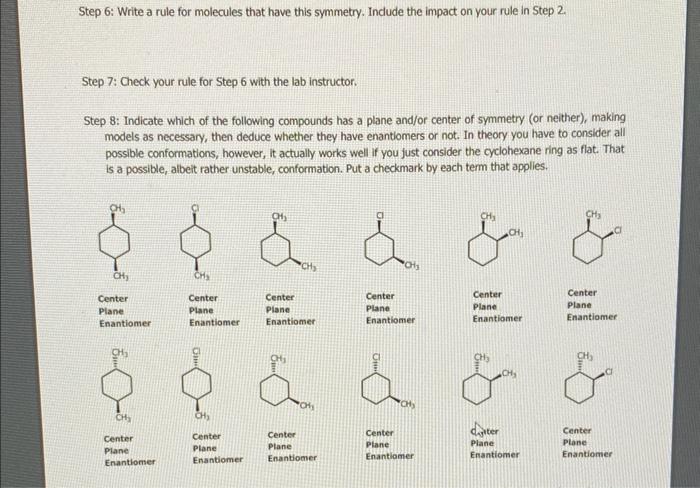

Step 6: Write a rule for molecules that have this symmetry. Indude the impact on your rule in Step 2. Step 7: Check your rule for Step 6 with the lab instructor. Step 8: Indicate which of the following compounds has a plane and/or center of symmetry (or neither), making models as necessary, then deduce whether they have enantiomers or not. In theory you have to consider all possible conformations, however, it actually works well if you just consider the cyclohexane ring as flat. That is a possible, albeit rather unstable, conformation. Put a checkmark by each term that applies. Center Piane Enantiomer Center Plane Enantiomer Center Plane Enantiomer Center Plane Center Plane Enantiomer Enantiomer Enantiomer Center Center Plane Enantiomer Part 5, Symmetry in Chemistry Background Knowledge In both inorganic and organic chemistry, symmetry is important because, as you will see, it can help us recognize when enantiomers exist, pairs of compounds that are mirror images of each other but that are not identical. Symmetry is a somewhat complicated sub-discipline of mathematics, but we will only look at two simple types of symmetry. Having a plane of symmetry mearis that a plane can be imagined splitting the molecule in such a way that the two halves are identical but mirror images of each other. Put more unambiguously, for every atom on one side of the plane, there is another atom equidistant on the other side that lies on a common line perpendicular to the plane. Having a center of symmetry means that there is a point in the molecule such that for every given atom there is another atom on a line that goes through the given atom and through the center of symmetry that is equidistant from the center of symmetry. Note that. molecules have many conformations, and each conformation has its own symmetry properties. However, in general, if a molecule has any conformation that has a plane or center of symmetry, we consider the molecule in general to have that symmetry. Procedure 5 Step 1: For the molecules below, determine whether each has 1) a plane of symmetry and/or 2) a center of symmetry. (The "Pair" designation is from Part 3.) Put a checkmark next to each symmetry that applies to that molecule, if either. Step 2: Compare these results with that in Part 3 and write a simple rule relating the presence of a plane of symmetry to the existence of enantiomers
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


