Question: Step 6: Write a rule for molecules that have this symmetry. Include the impact on your rule in Step 2. Step 7: Check your rule
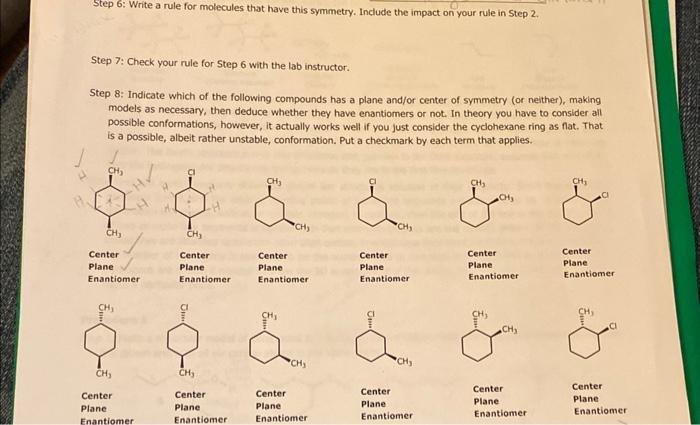

Step 6: Write a rule for molecules that have this symmetry. Include the impact on your rule in Step 2. Step 7: Check your rule for Step 6 with the lab instructor. Step 8: Indicate which of the following compounds has a plane and/or center of symmetry (or neither), making models as necessary, then deduce whether they have enantiomers or not. In theory you have to consider ail possible conformations, however, it actually works well if you just consider the cydohexane ring as flat. That is a possible, albeit rather unstable, conformation. Put a checkmark by each term that applies. Step 3: Build the molecule shown on the right (any colored ball for Br ). Question 1: Does it have a plane of symmetry? Question 2: Does it have a center of symmetry? Wes Step 4: Draw the mirror image of this molecule (cyclobutane already drawn), and then make a model of it as well. (You may have to cooperate with another group if you don't have enough atoms.) Step 5: Compare the two structures you built models of. Question 3: Are they the same or different, i.e., enantiomers? Step 6: Write a rule for molecules that have this symmetry. Indude the impact on your rule in step 2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


