Question: I need c!!! PLEASE HELP!!!! a) .193 b) 42.55 d) 16.8 e) 2.95 The gas phase reversible reaction A+B++D+ E is taking place in a
I need c!!! PLEASE HELP!!!!
a) .193
b) 42.55
d) 16.8
e) 2.95
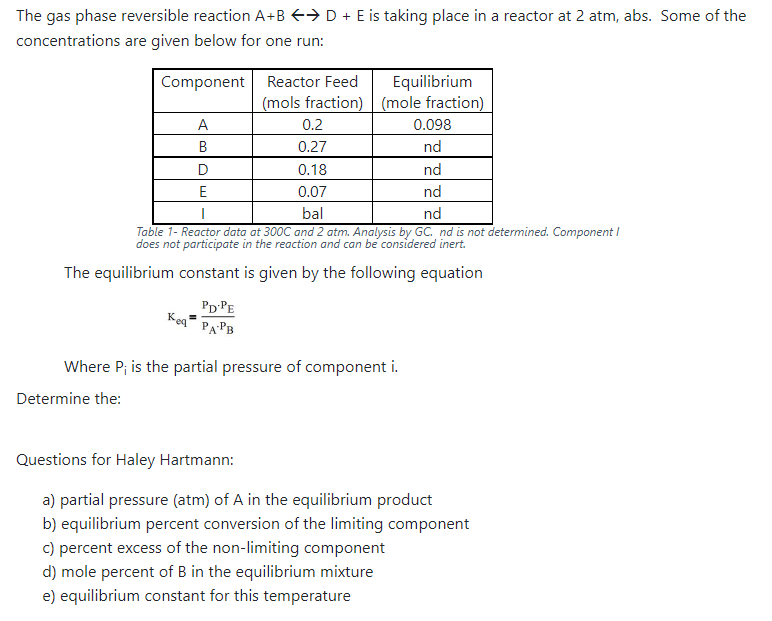
The gas phase reversible reaction A+B++D+ E is taking place in a reactor at 2 atm, abs. Some of the concentrations are given below for one run: Component Reactor Feed Equilibrium (mols fraction) (mole fraction) 0.2 0.098 B 0.27 nd D 0.18 nd E 0.07 nd | bal nd Table 1- Reactor data at 300C and 2 atm. Analysis by GC nd is not determined. Component! does not participate in the reaction and can be considered inert The equilibrium constant is given by the following equation p:PE PAPB Keq Where P; is the partial pressure of component i. Determine the: Questions for Haley Hartmann: a) partial pressure (atm) of A in the equilibrium product b) equilibrium percent conversion of the limiting component C) percent excess of the non-limiting component d) mole percent of B in the equilibrium mixture e) equilibrium constant for this temperature
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


