Question: i need calculations asap please!!! Data from your lab partner must be shared and collected during class time and signed of by your TA. Freezing
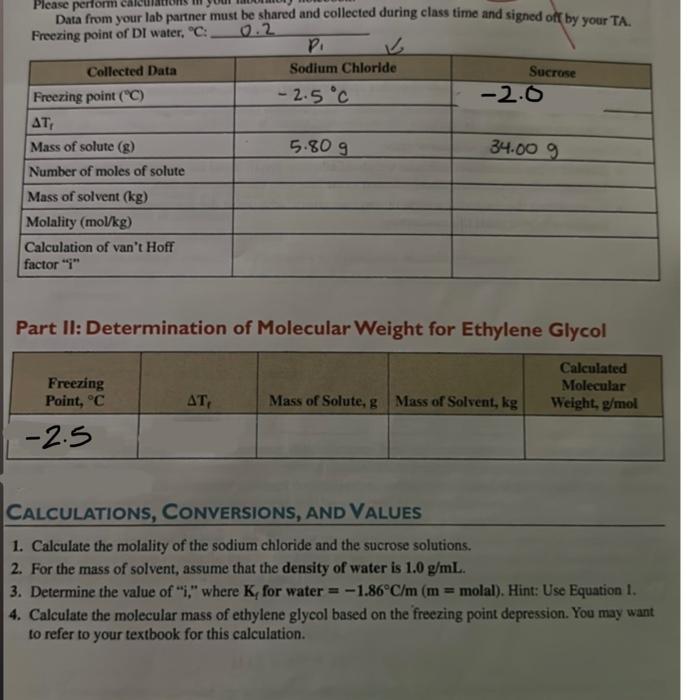
Data from your lab partner must be shared and collected during class time and signed of by your TA. Freezing point of DI water, C : 0.2 Part II: Determination of Molecular Weight for Ethylene Glycol Calculations, Conversions, and Values 1. Calculate the molality of the sodium chloride and the sucrose solutions. 2. For the mass of solvent, assume that the density of water is 1.0g/mL. 3. Determine the value of "i," where Kf for water =1.86C/m(m=molal). Hint: Use Equation I. 4. Calculate the molecular mass of ethylene glycol based on the freezing point depression. You may want to refer to your textbook for this calculation
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


