Question: I need code of part b only as text form.Part a and b is done on chegg i want answer of part b as text
 I need code of part b only as text form.Part a and b is done on chegg i want answer of part b as text which can be run on STANJAN (CEA RUN).
I need code of part b only as text form.Part a and b is done on chegg i want answer of part b as text which can be run on STANJAN (CEA RUN).
IM looking for best expert who can handle this task. i will give him best feedback.
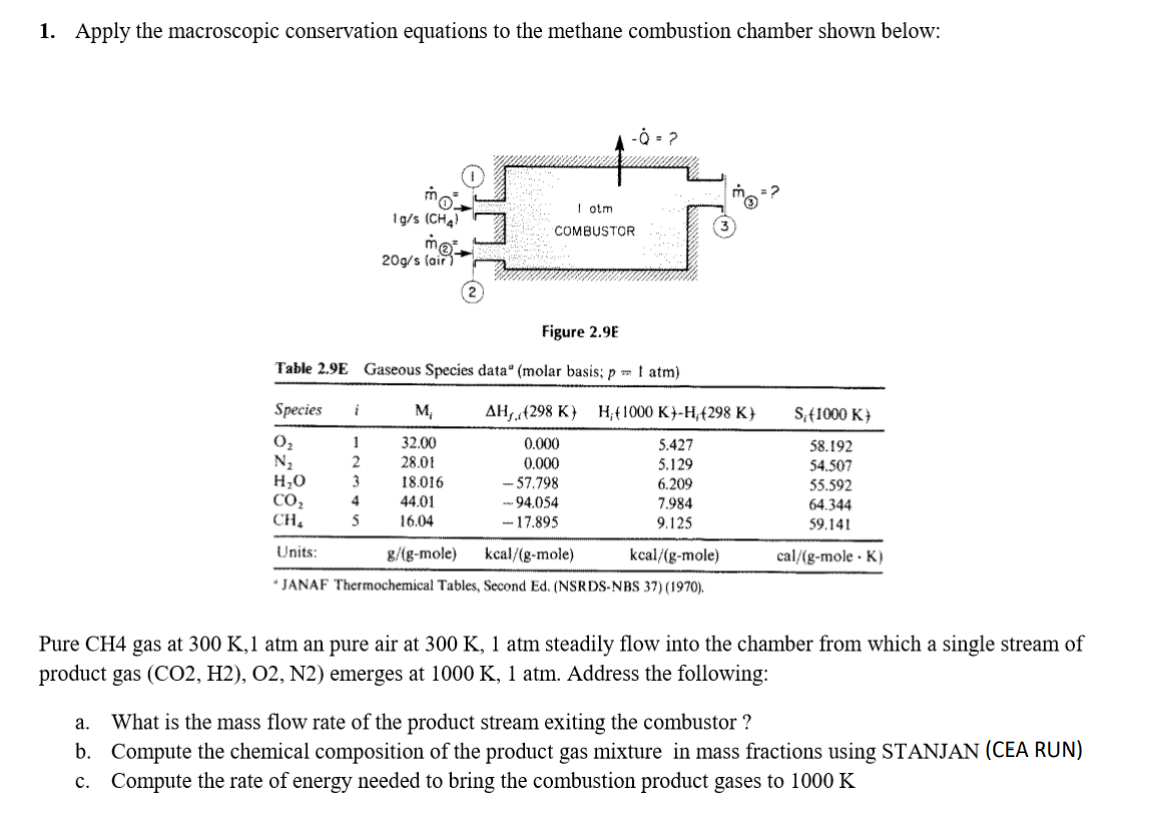
1. Apply the macroscopic conservation equations to the methane combustion chamber shown below: -02 I otm COMBUSTOR 1g/s (CHA) mo 20g/s fair Figure 2.9E Table 2.9E Gaseous Species data" (molar basis: p 1 atm) Species i M AH. 298 K) H;t1000 K -H (298 K S: 1000 K) 02 N2 HO CO2 CH, 1 2 3 4 S 32.00 28.01 18.016 44.01 16.04 0.000 0.000 -- 57.798 94.054 -17.895 5.427 5.129 6.209 7.984 9.125 58.192 54.507 55.592 64.344 59.141 cal/(g-mole .K) Units: g/(g-mole) kcal/(g-mole) kcal/(g-mole) JANAF Thermochemical Tables, Second Ed. (NSRDS-NBS 37) (1970). Pure CH4 gas at 300 K, 1 atm an pure air at 300 K, 1 atm steadily flow into the chamber from which a single stream of product gas (CO2, H2), O2, N2) emerges at 1000 K, 1 atm. Address the following: a. What is the mass flow rate of the product stream exiting the combustor ? b. Compute the chemical composition of the product gas mixture in mass fractions using STANJAN (CEA RUN) c. Compute the rate of energy needed to bring the combustion product gases to 1000 K
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


