Question: i need help filling out this chart from the data of my experiment 10. Complete the following table. You may attach a spreadsheet instead. If
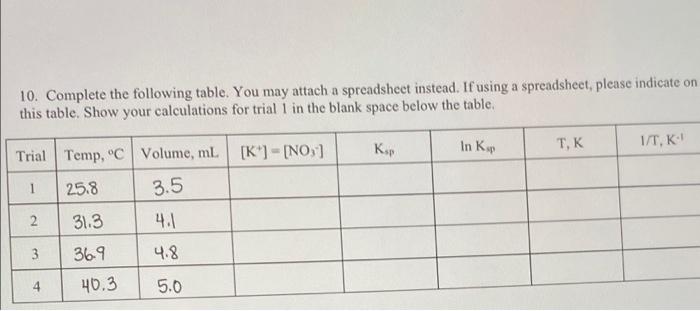
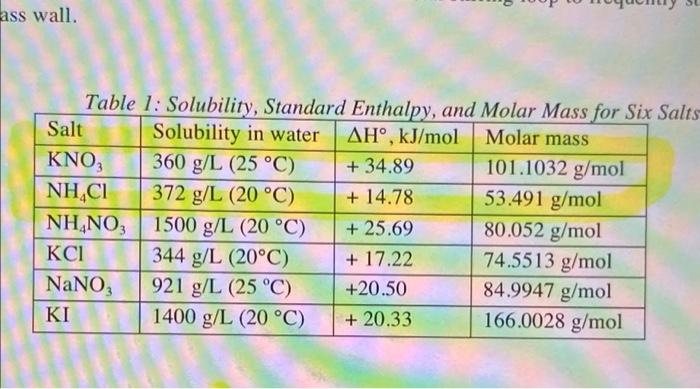
10. Complete the following table. You may attach a spreadsheet instead. If using a spreadsheet, please indicate on this table. Show your calculations for trial I in the blank space below the table, In Kup TEK 1/T, K Trial Temp. C Volume, ml [K"] = [NO, 1 1 25.8 3.5 2 31.3 4.1 369 4.8 3 4 40.3 5.0 ass wall. Table 1: Solubility, Standard Enthalpy, and Molar Mass for Six Salts Salt Solubility in water AH, kJ/mol Molar mass KNO, 360 g/L (25 C) + 34.89 101.1032 g/mol NH4Cl 372 g/L (20 C) + 14.78 53.491 g/mol NH NO3 1500 g/L (20 C) + 25.69 80.052 g/mol 344 g/L (20C) + 17.22 74.5513 g/mol NaNO 921 g/L (25 C) +20.50 84.9947 g/mol KI 1400 g/L (20 C) + 20.33 166.0028 g/mol 10. Complete the following table. You may attach a spreadsheet instead. If using a spreadsheet, please indicate on this table. Show your calculations for trial I in the blank space below the table, In Kup TEK 1/T, K Trial Temp. C Volume, ml [K"] = [NO, 1 1 25.8 3.5 2 31.3 4.1 369 4.8 3 4 40.3 5.0 ass wall. Table 1: Solubility, Standard Enthalpy, and Molar Mass for Six Salts Salt Solubility in water AH, kJ/mol Molar mass KNO, 360 g/L (25 C) + 34.89 101.1032 g/mol NH4Cl 372 g/L (20 C) + 14.78 53.491 g/mol NH NO3 1500 g/L (20 C) + 25.69 80.052 g/mol 344 g/L (20C) + 17.22 74.5513 g/mol NaNO 921 g/L (25 C) +20.50 84.9947 g/mol KI 1400 g/L (20 C) + 20.33 166.0028 g/mol
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


