Question: I need help filling out this table. Please show work for each trial and use the proper significant figures please. Procedure is the second picture.
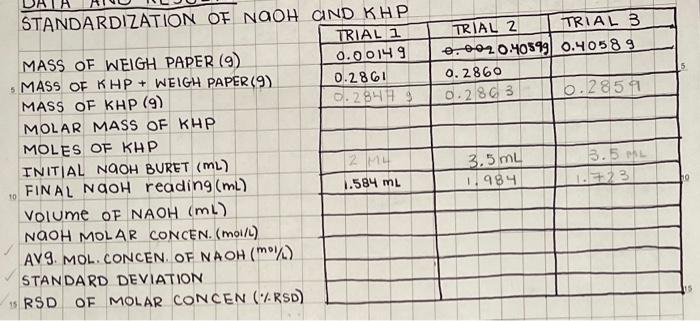

STANDARDIZATION OF NAOH GID KHP MASS OF WEIGH PAPER (9) - MASS OF KHP + WEIGH PAPER (9) MASS OF KHP ( 9 ) MOLAR MASS OF KHP MOLES OF KHP INITIAL NAOH BURET (mL) FINAL NAOH reading (mL) Volume of NAOH(ml) NOOH MOLAR CONCEN. (MOIL) AVS. MOL. CONCEN. OF NAOH ( STANDARD DEVIATION RSD of mOLAR CONCEN (\%RSD) \begin{tabular}{|l|l|l|} \hline TRIAL I & TR1AL 2 & TR1AL 3 \\ \hline 0.00149 & 0.2860.40599 & 0.40589 \\ \hline 0.2861 & 0.286 & \\ \hline 0.28479 & 0.2863 & 0.2859 \\ \hline & & \\ \hline & & \\ \hline & 3.5mL & 3.5mL \\ \hline 1.584mL & 1.984 & 1.723 \\ \hline & & \\ \hline & & \\ \hline & & \\ \hline & & \\ \hline & & \\ \hline \end{tabular} day, you will dilute a 7MNaOH solution to 100.0mL of approximately 0.7MNaOH. Then you will standardize the 0.7MNaOH with KHP and titrate HCl to the phenolphthalein end point to determine the unknown HCl concentration. Save the remaining standardized NaOH to use for the vinegar titration. On the next lab day, you will titrate both light and dark household vinegar samples to determine the amount of acetic acid in the vinegar solutions
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


