Question: I need help with question 3. Data Table 1. 83 #2 81 0.40) 0.4/2 Mass of KHP (e) 0.440 Initial buret reading (ml) 5.00 21.25
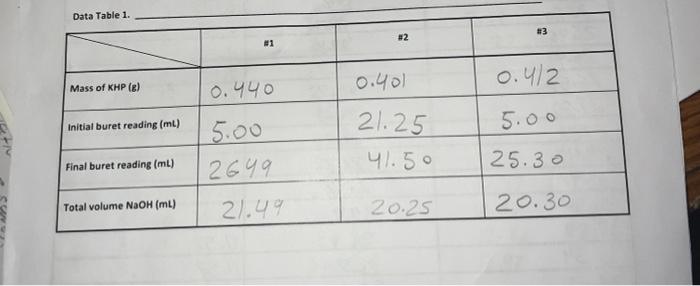
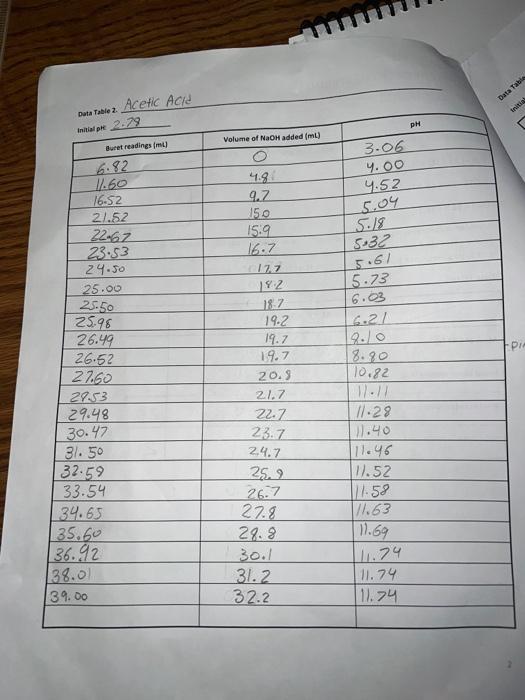
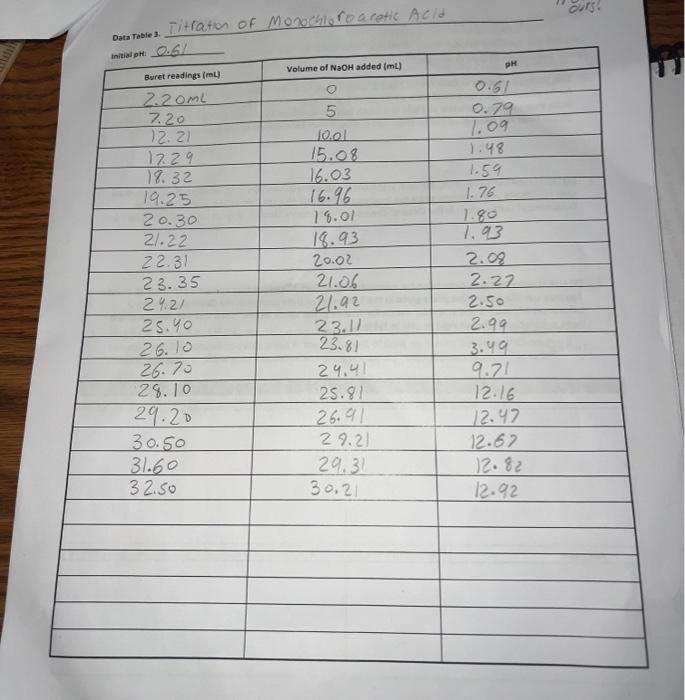

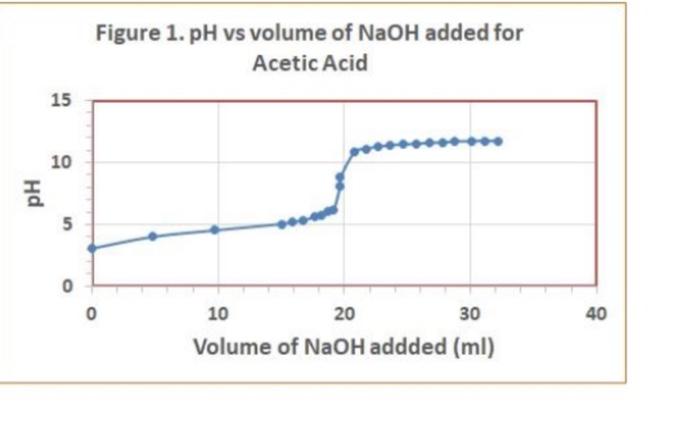
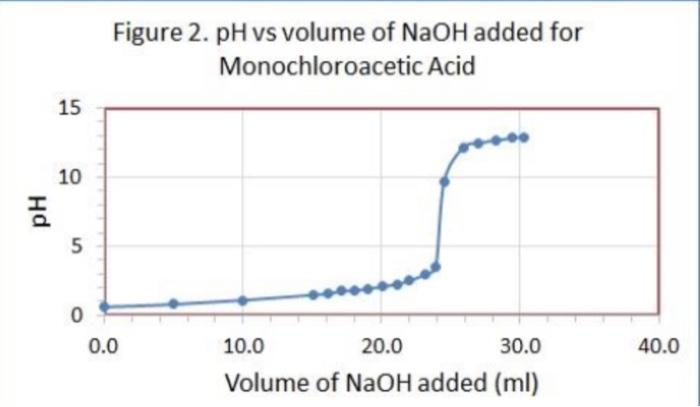
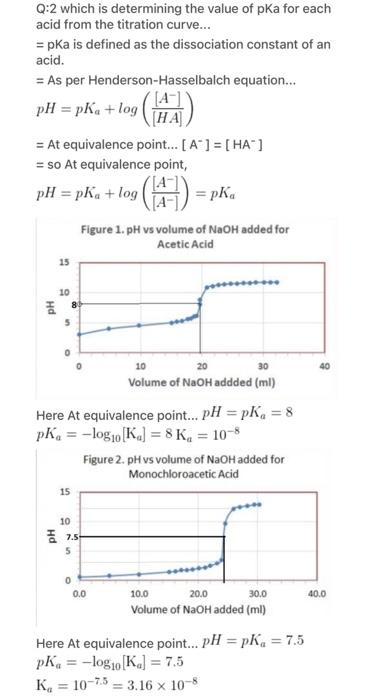
Data Table 1. 83 #2 81 0.40) 0.4/2 Mass of KHP (e) 0.440 Initial buret reading (ml) 5.00 21.25 41.50 Final buret reading (ml) 5.00 2649 21.49 25.30 Total volume NaOH (ml) 20.25 20.30 Data Table Ini Duta Table z Acetic Acid Initial pre 2.29 PH Volume of NaOH added (ml) Buret readings (m) 6.82 3.06 4.00 4.52 5.04 5.18 5:32 5.61 5.73 6.03 16.21 17.60 16.52 21.52 22.62 23.53 24.50 25.00 25.50 25.98 26.49 26.52 2790 24.53 29.48 30.47 31. 50 | 32.59 33.54 34.65 35.60 136.92 138.00 39.00 41.9 9.2 150 15.9 16.7 177 192 18.7 19.2 19.7 19.7 20.9 21.7 22.7 23.7 24.7 25.2 26.7 27.8 28.8 30. 31.2 32.2 8.90 10,82 17 11.28 11.40 11.46 11.52 111.58 76.63 11.69 111.74 11.79 111.74 ours. Data Tables Titration of Morochloroacetic Acid Initiat : 0.64 Volume of NaOH added (ml) M Buret readings (m) 0.61 0.79 1.09 1.48 1.59 1.76 2.20ML 7.20 12.21 17.29 18.32 19.25 20.30 21.22 22.31 23.35 29.21 25.40 26.10 5 fool 15.08 16.03 16.96 18.01 18.93 20.02 21.06 21.92 23.11 23.81 24.41 25.99 26.91 29.21 29.31 30.21 26.70 7.93 2.08 2.27 2.50 2.99 3.49 9.71 12.16 12.47 12.67 12.82 12.92 28.10 29.20 30.50 31.60 32.50 TREATMENT OF DATA (45 pts.) Calculate the following values Show all steps of your work, be sure to use the proper number of significant figures and include all units in your calculations. 1. (10) using your values in Data Tables 2 and 3. draw titration curves for both acids, plotting pH vs Volume of NaOH added. Each graph must have a numbered and catscriptive title les. Figure 1. Tale of Graph Scale your axes appropriately and add as botes with leses where appropriate). You should use a scatter plot in Excel and hand-draw the titration curve Staple your Excel graphs to the report sheet 2. (4) Determine the value of pK, for each acid from the titration curves. On your two titration curves, draw lines to indicate your logic and point out locations of interest. Use the calculation guide given in the Treatment of Data section in the General Chemistry 2 Laboratory Manual Report your results in Table 5. 3. (5) Use stoichiometry to determine the concentration of each NaOH solution compared to the KHP standard. You may use the calculation guide given in the Treatment of Data section in the General Chemistry 2 Laboratory Manual. Show your calculation for Trial #1 in the space below. Then find the average of the three calculations. Report your results in Table 4. Trial #1: Average: Figure 1. pH vs volume of NaOH added for Acetic Acid 15 10 Hd 5 0 0 20 40 10 30 Volume of NaOH addded (ml) Figure 2. pH vs volume of NaOH added for Monochloroacetic Acid 15 10 Hd 5 5 O 0 0.0 40.0 10.0 20.0 30.0 Volume of NaOH added (ml) Q:2 which is determining the value of pKa for each acid from the titration curve... = pka is defined as the dissociation constant of an acid. = As per Henderson-Hasselbalch equation... [ (). = At equivalence point... [ A ] = [HA] = so At equivalence point, pH = pka +10g (HA) pH = pka + 109 (43) = pku Figure 1. pH vs volume of NaOH added for Acetic Acid 15 10 8 5 10 20 30 40 Volume of NaOH addded (ml) Here At equivalence point... pH = p = 8 pk, = -log10 K.] = 8 K. = 10-8 Figure 2. pH vs volume of NaOH added for Monochloroacetic Acid 15 10 7.5 5 5 O 0.0 10.0 20.0 30.0 40.0 Volume of NaOH added (ml) Here At equivalence point... pH = pk. = 7.5 pk = -log10 (K) = 7.5 K = 10-7.5 = 3.16 x 10-8
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


