Question: I need help finding the conversion. The composition is .3507 HCl, 0.0396 O2, 0.3047 H2O, and 0.3047 Cl2 1) The following reaction takes place at
I need help finding the conversion. The composition is .3507 HCl, 0.0396 O2, 0.3047 H2O, and 0.3047 Cl2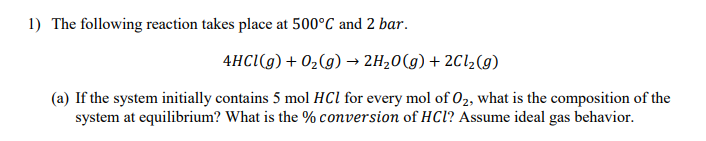
1) The following reaction takes place at 500C and 2 bar. 4HCl(g) + O2(g) 2H20(g) + 2C12(9) (a) If the system initially contains 5 mol HCl for every mol of Oz, what is the composition of the system at equilibrium? What is the % conversion of HCl? Assume ideal gas behavior
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


