Question: i need help for the three questions A solution contains 0.113M ammonium bromide and 0.326M ammonia. The pH of this solution is The compound ethylamine
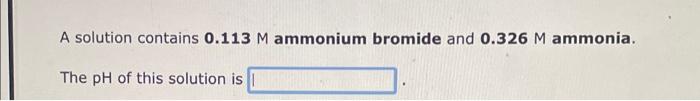


A solution contains 0.113M ammonium bromide and 0.326M ammonia. The pH of this solution is The compound ethylamine is a weak base like ammonia. A solution contains 0.172M C2H5NH3+and 0.355M ethylamine, C2H5NH2. The pH of this solution is The compound methylamine is a weak base like ammonia. A solution contains 0.428 MCH3NH3+and 0.426M methylamine, CH3NH2. The pH of this solution is
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


