Question: i need help for the three questions Consider the reaction: 2CO(g)+2NO(g)2CO2(g)+N2(g) Using standard absolute entropies at 298K, calculate the entropy change for the system when
i need help for the three questions 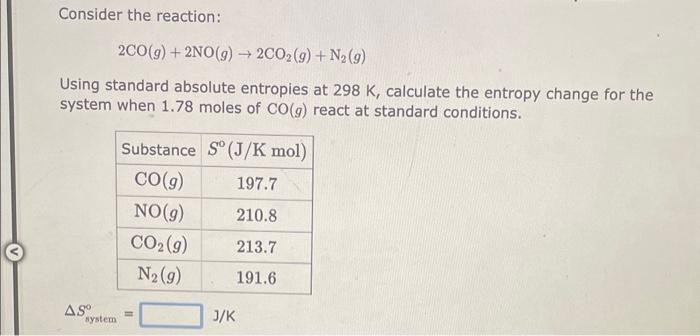
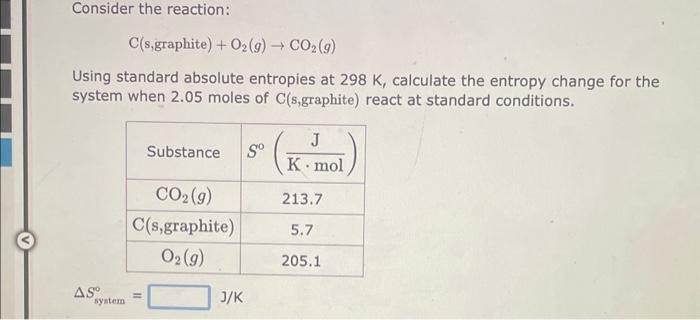
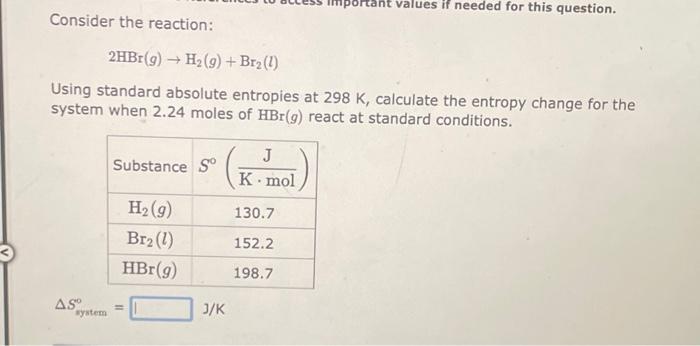



Consider the reaction: 2CO(g)+2NO(g)2CO2(g)+N2(g) Using standard absolute entropies at 298K, calculate the entropy change for the system when 1.78 moles of CO(g) react at standard conditions. Ssystem=J/K Consider the reaction: C(s,graphite)+O2(g)CO2(g) Using standard absolute entropies at 298K, calculate the entropy change for the system when 2.05 moles of C(s, graphite) react at standard conditions. Ssyatem=J/K Consider the reaction: 2HBr(g)H2(g)+Br2(l) Using standard absolute entropies at 298K, calculate the entropy change for the system when 2.24 moles of HBr(g) react at standard conditions. Ssystem=
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


