Question: i need help for the three questions The maximum amount of silver sulfide that will dissolve in a 0.126M potassium sulfide solution is M. No
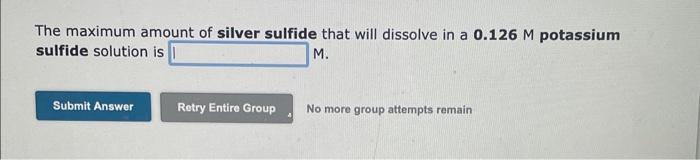
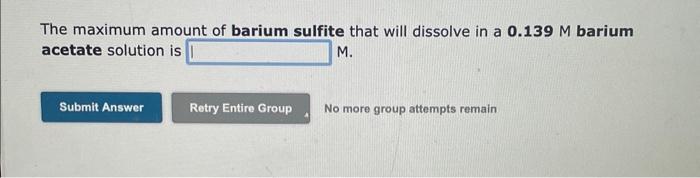

The maximum amount of silver sulfide that will dissolve in a 0.126M potassium sulfide solution is M. No more group attempts remain The maximum amount of barium sulfite that will dissolve in a 0.139M barium acetate solution is M. No more group attempts remain The molar solubility of lead sulfate in a 0.153M sodium sulfate solution is M. The maximum amount of silver sulfide that will dissolve in a 0.126M potassium sulfide solution is M. No more group attempts remain The maximum amount of barium sulfite that will dissolve in a 0.139M barium acetate solution is M. No more group attempts remain The molar solubility of lead sulfate in a 0.153M sodium sulfate solution is M
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


