Question: please do all no work needed please thank you The equilibrium constant, Kp, for the following reaction is 0.636 at 600,K. COCl2(g)CO(g)+Cl2(g) If an equilibrium


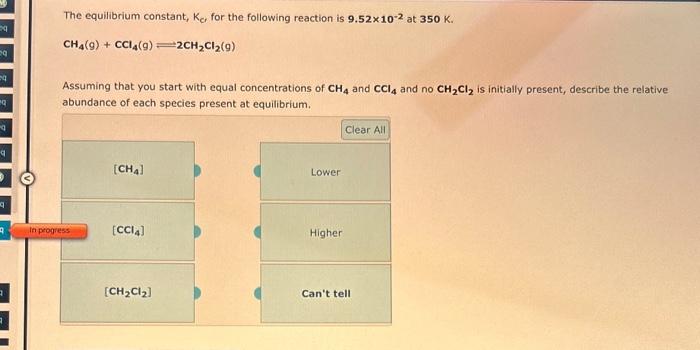
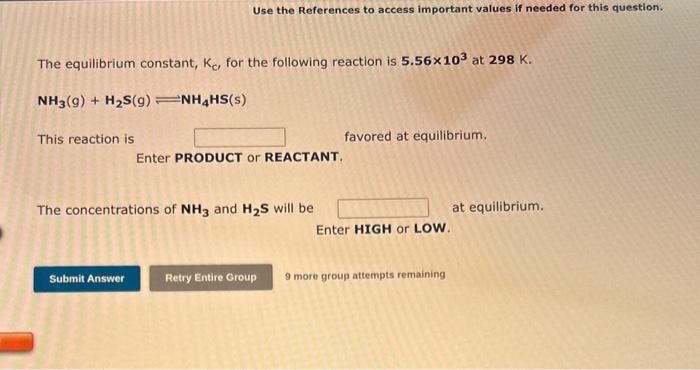
The equilibrium constant, Kp, for the following reaction is 0.636 at 600,K. COCl2(g)CO(g)+Cl2(g) If an equilibrium mixture of the three gases in a 13.9L container at 600.K contains COCl2 at a pressure of 0.584 atm and CO ot a pressure of 0.793atm, the equilibrium partial pressure of Cl2 is atm. 9 more group attempte rempining Consider the following reaction: CoCl2(g)CO(g)+Cl2(g) If 2.50103 moles of COCl2,0.225 moles of CO, and 0.270 moles of Cl2 are at equilibrium in a 16.0L container at 781K, the value of the equilibrium constant, Kp, is The equilibrium constant, Kc, for the following reaction is 9.52102 at 350K. CH4(g)+CCl4(g)2CH2Cl2(g) Assuming that you start with equal concentrations of CH4 and CCl4 and no CH2Cl2 is initially present, describe the relative abundance of each species present at equilibrium. Use the References to access important values if needed for this question. The equilibrium constant, Kc, for the following reaction is 5.56103 at 298K. NH3(g)+H2S(g)NH4HS(s) This reaction is favored at equilibrium. Enter PRODUCT or REACTANT. The concentrations of NH3 and H2S will be at equilibrium. Enter HIGH or LOW. 9 more group attempts remaining
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


