Question: i need help for the three questions Use the standard reduction potentials located in the 'Tables' linked above to calculate the equilibrium constant for the
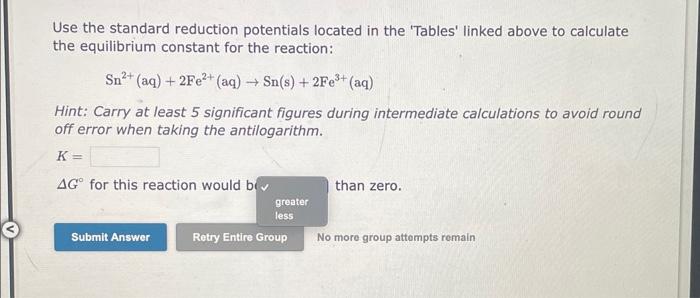
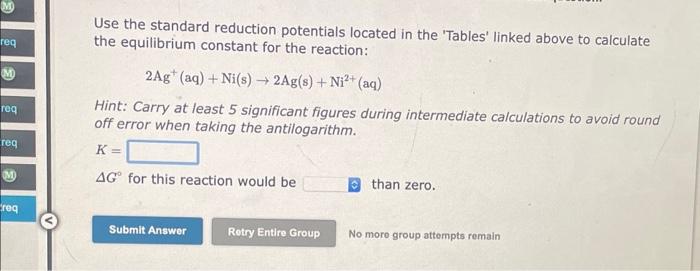
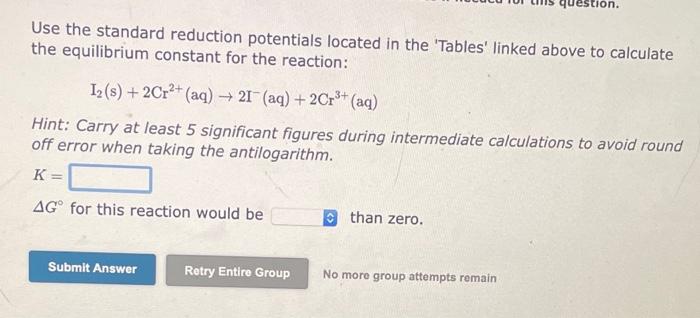
Use the standard reduction potentials located in the 'Tables' linked above to calculate the equilibrium constant for the reaction: Sn2+(aq)+2Fe2+(aq)Sn(s)+2Fe3+(aq) Hint: Carry at least 5 significant figures during intermediate calculations to avoid round off error when taking the antilogarithm. K= G for this reaction would bi than zero. No more group attompts remain Use the standard reduction potentials located in the 'Tables' linked above to calculate the equilibrium constant for the reaction: 2Ag+(aq)+Ni(s)2Ag(s)+Ni2+(aq) Hint: Carry at least 5 significant figures during intermediate calculations to avoid round off error when taking the antilogarithm. K= G for this reaction would be than zero. No moro group attempts remain Use the standard reduction potentials located in the 'Tables' linked above to calculate the equilibrium constant for the reaction: I2(s)+2Cr2+(aq)2I(aq)+2Cr3+(aq) Hint: Carry at least 5 significant figures during intermediate calculations to avoid round off error when taking the antilogarithm. K= G for this reaction would be than zero. No more group attempts remain
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


